Flaccidoside IICAS# 140694-19-5 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 140694-19-5 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C59H96O25 | M.Wt | 1205.39 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Flaccidoside II Dilution Calculator

Flaccidoside II Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.8296 mL | 4.148 mL | 8.2961 mL | 16.5921 mL | 20.7402 mL |
| 5 mM | 0.1659 mL | 0.8296 mL | 1.6592 mL | 3.3184 mL | 4.148 mL |
| 10 mM | 0.083 mL | 0.4148 mL | 0.8296 mL | 1.6592 mL | 2.074 mL |
| 50 mM | 0.0166 mL | 0.083 mL | 0.1659 mL | 0.3318 mL | 0.4148 mL |
| 100 mM | 0.0083 mL | 0.0415 mL | 0.083 mL | 0.1659 mL | 0.2074 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Arundoin
Catalog No.:BCX1389
CAS No.:4555-56-0
- γ-Linolenic acid
Catalog No.:BCX1388
CAS No.:506-26-3
- Linoleic acid sodium salt
Catalog No.:BCX1387
CAS No.:822-17-3
- Methyl Eicosapentaenoate
Catalog No.:BCX1386
CAS No.:2734-47-6
- Eicosapentaenoic acid ethyl ester
Catalog No.:BCX1385
CAS No.:86227-47-6
- Docosahexaenoic acid methyl ester
Catalog No.:BCX1384
CAS No.:2566-90-7
- Docosahexaenoic acid ethyl ester
Catalog No.:BCX1383
CAS No.:84494-72-4
- Ethyl oleate
Catalog No.:BCX1382
CAS No.:111-62-6
- Methyl arachidonate
Catalog No.:BCX1381
CAS No.:2566-89-4
- Ethyl Arachidonate
Catalog No.:BCX1380
CAS No.:1808-26-0
- Arachidic acid
Catalog No.:BCX1379
CAS No.:506-30-9
- Palmitoleic acid
Catalog No.:BCX1378
CAS No.:373-49-9
- Peruvoside
Catalog No.:BCX1391
CAS No.:1182-87-2
- Thevetin A
Catalog No.:BCX1392
CAS No.:37933-66-7
- 3-O-Acetylbufotalin
Catalog No.:BCX1393
CAS No.:4029-69-0
- Smilagenin
Catalog No.:BCX1394
CAS No.:126-18-1
- Elaidic acid
Catalog No.:BCX1395
CAS No.:112-79-8
- 7-Hydroxyisoflavone
Catalog No.:BCX1396
CAS No.:13057-72-2
- 7α-Hydroxysarsasapogenin
Catalog No.:BCX1397
CAS No.:220832-70-2
- Tapinarof
Catalog No.:BCX1398
CAS No.:79338-84-4
- Senecionine acetate
Catalog No.:BCX1399
CAS No.:126642-77-1
- 4,3',5'-Trihydroxyresveratrol
Catalog No.:BCX1400
CAS No.:637776-83-1
- 7β-Hydroxyganoderenic acid F
Catalog No.:BCX1401
CAS No.:1245946-62-6
- 4-Methoxy-2-[(6-O-β-D-xylopyranosyl-β-D-glucopyranosyl)oxy]benzaldehyde
Catalog No.:BCX1402
CAS No.:140484-68-0
Flaccidoside II ameliorates collagen-induced arthritis in mice.[Pubmed:32360348]
Eur J Pharmacol. 2020 Aug 5;880:173155.
Flaccidoside II (FLA II), the primary active constituent from Anemone flaccida rhizome, was proven to exert therapeutic effect against collagen-induced arthritis (CIA). In this study, a research based on the CIA mouse model was carried out in order to elucidate its therapeutic mechanisms preliminarily. The mice were immunized with porcine type-II collagen to induce CIA and administrated intragastrically with FLA II daily from day 7-42 of the first collagen immunization. The arthritis scores as reflected by the severity of paw swelling and erythema were significantly reduced in FLA II (32 mg/kg) from day 33 onwards. On day 42, the joints of FLA II-treated mice exhibited obvious reductions of inflammatory cells infiltration, synovial hyperplasia and bone destruction. When the concentration of FLA II was no less than 40 nmol/ml, the treatment notably inhibited T and B lymphocyte proliferative responses. As compared to the model group, in FLA II groups, the serum levels of pro-inflammatory cytokines (IL-1beta, IL-6 and TNF-alpha) were significantly decreased while those of Th2 type cytokines (IL-4 and IL-10) were clearly enhanced. In addition, FLA II treatment showed little regulatory effect on the levels of Th1 type cytokines (IFN-gamma and IL-2). The severity of mice CIA was improved by FLA II, further confirming its potential value for the safe treatment of rheumatoid arthritis. The main mechanisms likely involve the inhibition of lymphocyte proliferation and pro-inflammatory cytokine secretion, and the modulation of Th1/Th2-related cytokine balance in CIA.
A two-step approach for systematic identification and quality evaluation of wild and introduced Anemone flaccida Fr. Schmidt (Di Wu) based on DNA barcode and UPLC-QTOF-MS/MS.[Pubmed:32025771]
Anal Bioanal Chem. 2020 Mar;412(8):1807-1816.
Herbal materials have both medicinal and commercial values. As such, accurate species and content identification and verification are necessary to ensure the safe and effective use for medical and commodity purposes. Herein, we introduce a two-step approach for systematic identification and quality evaluation of wild and introduced Anemone flaccida Fr. Schmidt (aka Di Wu) using DNA barcode and ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS/MS). To begin, a precise and rapid identification method based on internal transcribed spacer 2 (ITS2) sequence was developed to ensure the authenticity of 'Di Wu' species. Next, the major active components were fully characterized utilizing a targeted profile of oleanane-type triterpenoid saponins, which was established via UPLC-QTOF-MS/MS. As a result, 34 oleanane-type triterpenoid saponins were identified or characterized in 'Di Wu.' The qualitative and relative quantitative analysis showed obvious differences between wild and introduced 'Di Wu.' Furthermore, dynamic changes in the contents of triterpenoid saponins throughout various harvesting periods were clearly explained and mid-April was identified as the appropriate harvest time. Moreover, results indicate that the contents of five main saponins (anhuienoside E, glycosideSt-I4a, hemsgiganoside B, Flaccidoside II, and hederasaponin B) are more appropriate as a quality evaluation indicator than the current quality standard. The two-step approach provides a suitable strategy to evaluate the genuine quality of wild and introduced 'Di Wu,' and can be applied to the targeted analysis of other triterpenoid saponin analogues for quality evaluation. Graphical Abstract .
Hypouricemic effect of flaccidoside II in rodents.[Pubmed:27771848]
J Nat Med. 2017 Jan;71(1):329-333.
To investigate the effect of Flaccidoside II on the serum uric acid levels in hyperuricemic rodents. Both mice and rats were injected intraperitoneally with potassium oxonate to induce hyperuricemia. Different dosages of Flaccidoside II were orally administrated to hyperuricemic and normal rodents for 7 days, respectively. Liver xanthine oxidase (XOD) activities in hyperuricemic mice were determined using the colorimetric method. Acute toxicity of Flaccidoside II was also evaluated in mice. Allopurinol, as a positive control, was administered under the same treatment scheme. The results showed that Flaccidoside II (32, 16 and 8 mg/kg) could significantly lower serum uric acid levels in hyperuricemic mice. Flaccidoside II (24, 12 and 6 mg/kg) could also markedly lower serum uric acid levels in hyperuricemic rats. However, unlike allopurinol, oral administration of Flaccidoside II did not produce any observable hypouricemic effect in normal animals. Flaccidoside II at the dose of 32 mg/kg significantly suppressed XOD activities in the liver of hyperuricemic mice, while at doses of 16 and 8 mg/kg Flaccidoside II did not show a significant effect on XOD activities. In addition, Flaccidoside II (300 mg/kg) has no or less toxicity than allopurinol in mice. These findings demonstrate that Flaccidoside II exhibits anti-hyperuricemic activity in hyperuricemic animals.
Triterpenoid saponin flaccidoside II from Anemone flaccida triggers apoptosis of NF1-associated malignant peripheral nerve sheath tumors via the MAPK-HO-1 pathway.[Pubmed:27103823]
Onco Targets Ther. 2016 Apr 5;9:1969-79.
Malignant peripheral nerve sheath tumors (MPNSTs) are highly aggressive soft tissue neoplasms that are extremely rare and are frequently associated with neurofibromatosis type 1 patients. MPNSTs are typically fatal, and there is no effective treatment so far. In our previous study, we showed that Flaccidoside II, one of the triterpenoid saponins isolated from Anemone flaccida Fr. Schmidt, has antitumor potential by inducing apoptosis. In the present study, we found that Flaccidoside II inhibits proliferation and facilitates apoptosis in MPNST cell lines ST88-14 and S462. Furthermore, this study provides a mechanism by which the downregulation of heme oxygenase-1 via extracellular signal-regulated kinase-1/2 and p38 mitogen-activated protein kinase pathways is involved in the apoptotic role of Flaccidoside II. This study suggested the potential of Flaccidoside II as a novel pharmacotherapeutic approach for MPNSTs.
Crude triterpenoid saponins from Anemone flaccida (Di Wu) exert anti-arthritic effects on type II collagen-induced arthritis in rats.[Pubmed:26213566]
Chin Med. 2015 Jul 25;10:20.
BACKGROUND: Anemone flaccida Fr . Schmidt (Ranunculaceae) (Di Wu in Chinese) is used to treat punch injury and rheumatoid arthritis (RA). However, the active compounds and underlying mechanism of action mediating the anti-arthritic effects of A. flaccida remain unclear. This study aims to evaluate the underlying action mechanism of A. flaccida crude triterpenoid saponins (AFS) on RA using a type II collagen (CII)-induced arthritis (CIA) rat model, and to assess the anti-inflammatory effects of the main active compounds of AFS, namely Flaccidoside II, anhuienoside E, glycoside St-I4a, hemsgiganoside B, hederasaponin B, and 3-O-alpha-l-rhamnopyranosyl (1 --> 2)-beta-d-glucopyranosyl oleanolic acid 28-O-beta-d-glucopyranosyl (1 --> 6)-beta-d-glucopyranosyl ester. METHODS: Male Wistar rats (n = 50) were randomly separated into five groups (n = 10) and immunized by CII injection. AFS (200 or 400 mg/kg) and dexamethasone were orally administered for 30 days after establishing the model. The arthritis severity was assessed by paw volume using a plethysmometer. After 30 days of treatment, the right hind paws of the rats were obtained. Paw histology was analyzed by hematoxylin and eosin staining, and radiologic imaging was performed by micro-computed tomography. MTT assays were used to evaluate the cytotoxicity of AFS and its main compounds in RAW264.7 cells. Enzyme-linked immunosorbent assay kits were used to measure interleukin (IL)-6 and tumor necrosis factor (TNF)-alpha in serum and supernatants from AFS- and main AFS compound-treated RAW264.7 cells stimulated by lipopolysaccharide (LPS). RESULTS: Anemone flaccida crude triterpenoid saponins inhibited redness and swelling of the right hind paw in the CIA model. Radiological and histological examinations indicated that inflammatory responses were reduced by AFS treatment. Moreover, comparing with untreated rats, serum TNF-alpha (P = 0.0035 and P < 0.001) and IL-6 (P = 0.0058 and P = 0.0087) were lower in AFS-treated CIA rats at the dose of 200 and 400 mg/kg/day. AFS and its main compounds, including hederasaponin B, Flaccidoside II, and hemsgiganoside B, significantly inhibited TNF-alpha (P = 0.0022, P = 0.013, P = 0.0015, and P = 0.016) and IL-6 (P = 0.0175, P < 0.001, P < 0.001, and P < 0.001) production in LPS-treated RAW264.7 cells, respectively. CONCLUSIONS: Anemone flaccida crude triterpenoid saponins and its main bioactive components, including hederasaponin B, Flaccidoside II, and hemsgiganoside B, decreased pro-inflammatory cytokine levels in a CIA rat model and LPS-induced RAW264.7 cells.
The Antitumor Effects of Triterpenoid Saponins from the Anemone flaccida and the Underlying Mechanism.[Pubmed:24191167]
Evid Based Complement Alternat Med. 2013;2013:517931.
Anemone flaccida Fr. Schmidt, a family of ancient hopanoids, have been used as traditional Asian herbs for the treatments of inflammation and convulsant diseases. Previous study on HeLa cells suggested that triterpenoid saponins from Anemone flaccida Fr. Schmidt may have potential antitumor effect due to their apoptotic activities. Here, we confirmed the apoptotic activities of the following five triterpenoid saponins: glycoside St-I4a (1), glycoside St-J (2), anhuienoside E (3), hedera saponin B (4), and Flaccidoside II (5) on human BEL-7402 and HepG2 hepatoma cell lines, as well as the model of HeLa cells treated with lipopolysaccharide (LPS). We found that COX-2/PGE2 signaling pathway, which plays key roles in the development of cancer, is involved in the antitumor activities of these saponins. These data provide the evidence that triterpenoid saponins can induce apoptosis via COX-2/PGE2 pathway, implying a preventive role of saponins from Anemone flaccida in tumor.
Triterpenoid saponins from Anemone flaccida induce apoptosis activity in HeLa cells.[Pubmed:19219723]
J Asian Nat Prod Res. 2009;11(2):122-7.
Five triterpenoid saponins were isolated from Anemone flaccida Fr. Schmidt. Their structures were identified as glycoside St-I4a (1), glycoside St-J (2), anhuienoside E (3), hederasaponin B (4), and Flaccidoside II (5). Compounds 1-2 were isolated from Anemone family for the first time, and compounds 3-4 were isolated from this plant for the first time. The inhibitory effects of saponins on proliferation of HeLa cells were studied by MTT assay, the apoptosis-induction activity was observed by cell-cycle analysis and caspase-3 expression assay. The antitumor activities of the saponins were ranked in the following order: 5 > 3 > 4 > 1 > 2. The data presented here indicated that naturally occurring triterpenoid saponins can be regarded as excellent structures for the potential development of new anticancer agents.
Synthesis of flaccidoside II, a bidesmosidic triterpene saponin isolated from Chinese folk medicine Di Wu.[Pubmed:18070618]
Carbohydr Res. 2008 Feb 25;343(3):462-9.
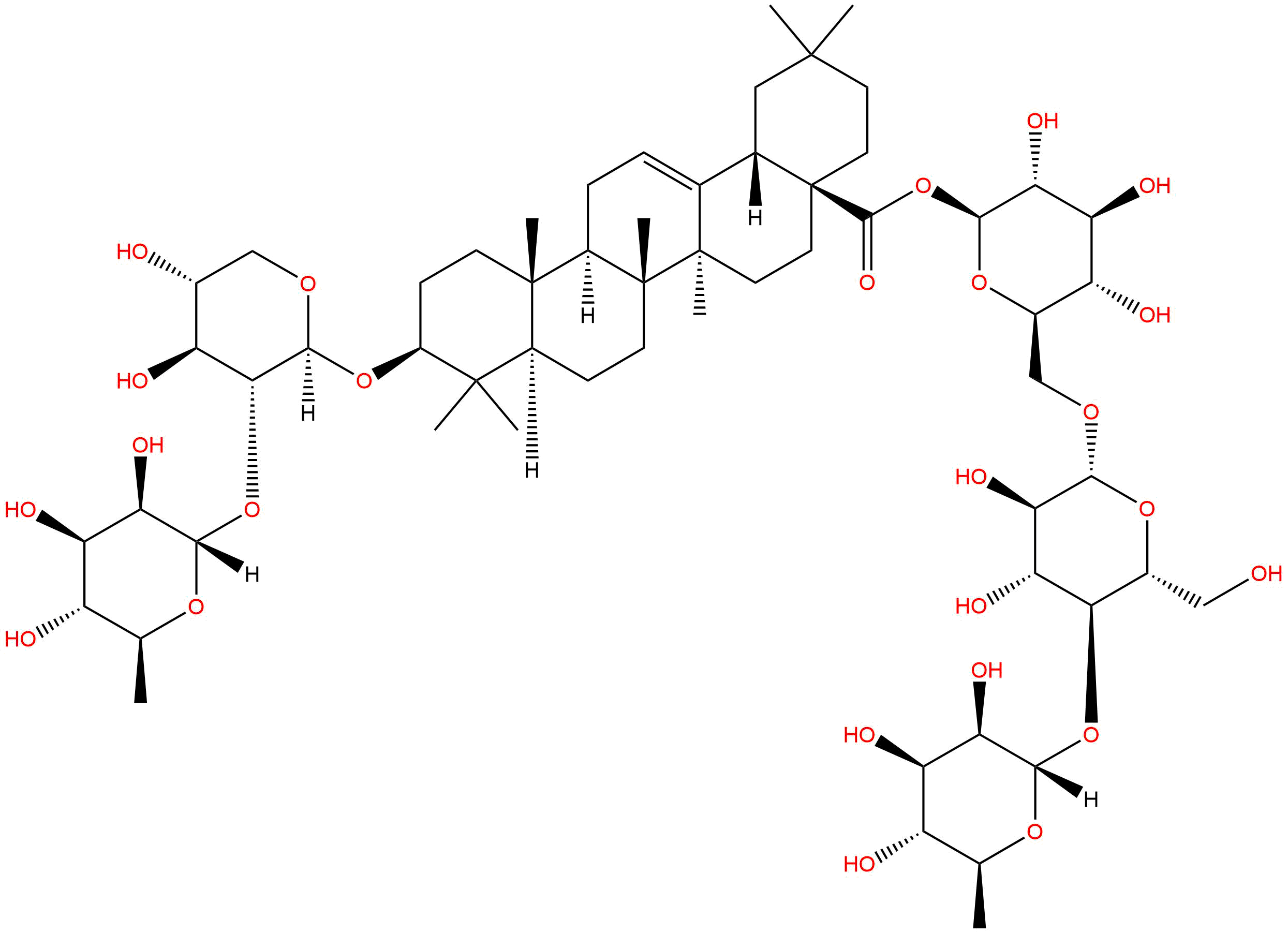
A total synthesis of Flaccidoside II, 3-O-alpha-L-rhamnopyranosyl-(1-->2)-beta-D-xylopyranosyloleanolic acid 28-O-alpha-L-rhamnopyranosyl-(1-->4)-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranoside, isolated from Chinese folk medicine Di Wu, has been accomplished from building blocks isopropyl 2-O-acetyl-3,4-di-O-benzoyl-1-thio-beta-D-xylopyranoside, 2,3,4-tri-O-benzoyl-alpha-L-rhamnopyranosyl trichloroacetimidate, oleanolic acid trityl ester, ethyl 2,3-di-O-acetyl-6-O-benzoyl-1-thio-beta-D-glucopyranoside and 4-methoxyphenyl 2,3,4-tri-O-acetyl-beta-D-glucopyranoside. The use of a partially protected thioglycosyl donor significantly simplified the synthesis of the target saponin.
[Studies on chemical constituents from Anemone anhuiensis Y. K. Yang N. Wang et W. C. Ye].[Pubmed:12776429]
Zhongguo Zhong Yao Za Zhi. 2001 Sep;26(9):612-4.
OBJECTIVE: To investigate the constituents from the roots of Anemone anhuiensis. METHOD: To isolate chemical constituents, using solvent extraction together with column chromatography, FAB-MS and NMR methods were employed for strutural elucidation. RESULT AND CONCLUSION: Three compounds were isolated and elucidated as oleanolic acid 28-O-alpha-L-rhamnopyranosyl-(1-->4)- beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyransyl ester (cussonoside B), 3-O-alpha-L-rhamnopyranosyl-(1-->2)-beta-D- xylopyranosyl oleanolic acid 28-O-alpha-L-rhamnopyranosyl-(1-->4)-beta-D- glucopyranosyl-(1-->6)-beta-D-glucopyransyl ester (Flaccidoside II) and 3-O-beta-D-glucopyranosyl-(1-->2)-beta-D-xylopyranosyl oleanolic acid 28-O-alpha-L-rhamnopyranosyl-(1-->4)-beta-D-glucopyranosyl- (1-->6)-beta-D-glucopyransyl ester (Flaccidoside III), respectively. All of them were isolated from the plant for the first time.
Two New Oleanane Saponins from Anemone flaccida.[Pubmed:17226205]
Planta Med. 1991 Dec;57(6):572-4.
Two new oleanane saponins, named Flaccidoside II and III, were isolated from the rhizome of ANEMONE FLACCIDA Fr. Schmidt. On the basis of spectroscopic analysis and chemical transformation their structures were elucidated as 3- O-[alpha- L-rhamnopyranosyl-(1-->2)-beta- D-xylopyranosyl]-oleanolic acid 28- O-[alpha- L-rhamnopyranosyl-(1-->4)-beta- D-glucopyranosyl-(1-->6)-beta- D-glucopyranoside] and 3- O-[beta- D-glucopyranosyl-(1-->2)-beta- D-xylopyranosyl]-oleanolic acid 28- O-[alpha- L-rhamnopyranosyl-(1-->4)-beta- D-glucopyranosyl-(1-->6)-beta- D-glucopyranoside].


