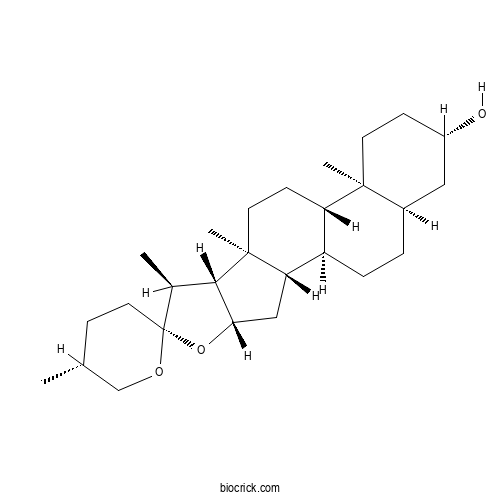
SmilageninCAS# 126-18-1 |
- Sarsasapogenin
Catalog No.:BCN1269
CAS No.:126-19-2
- Tigogenin
Catalog No.:BCN5327
CAS No.:77-60-1
- Sarsaponin
Catalog No.:BCN8293
CAS No.:82597-74-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 126-18-1 | SDF | Download SDF |
| PubChem ID | 91439.0 | Appearance | Powder |
| Formula | C27H44O3 | M.Wt | 416.65 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Synonyms | Isosarsasapogenin; Cogane; Esmilagenin | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2S,4S,5'R,6R,7S,8R,9S,12S,13S,16S,18R)-5',7,9,13-tetramethylspiro[5-oxapentacyclo[10.8.0.02,9.04,8.013,18]icosane-6,2'-oxane]-16-ol | ||
| SMILES | CC1CCC2(C(C3C(O2)CC4C3(CCC5C4CCC6C5(CCC(C6)O)C)C)C)OC1 | ||
| Standard InChIKey | GMBQZIIUCVWOCD-UQHLGXRBSA-N | ||
| Standard InChI | InChI=1S/C27H44O3/c1-16-7-12-27(29-15-16)17(2)24-23(30-27)14-22-20-6-5-18-13-19(28)8-10-25(18,3)21(20)9-11-26(22,24)4/h16-24,28H,5-15H2,1-4H3/t16-,17+,18-,19+,20-,21+,22+,23+,24+,25+,26+,27-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Smilagenin Dilution Calculator

Smilagenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4001 mL | 12.0005 mL | 24.001 mL | 48.0019 mL | 60.0024 mL |
| 5 mM | 0.48 mL | 2.4001 mL | 4.8002 mL | 9.6004 mL | 12.0005 mL |
| 10 mM | 0.24 mL | 1.2 mL | 2.4001 mL | 4.8002 mL | 6.0002 mL |
| 50 mM | 0.048 mL | 0.24 mL | 0.48 mL | 0.96 mL | 1.2 mL |
| 100 mM | 0.024 mL | 0.12 mL | 0.24 mL | 0.48 mL | 0.6 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-O-Acetylbufotalin
Catalog No.:BCX1393
CAS No.:4029-69-0
- Thevetin A
Catalog No.:BCX1392
CAS No.:37933-66-7
- Peruvoside
Catalog No.:BCX1391
CAS No.:1182-87-2
- Flaccidoside II
Catalog No.:BCX1390
CAS No.:140694-19-5
- Arundoin
Catalog No.:BCX1389
CAS No.:4555-56-0
- γ-Linolenic acid
Catalog No.:BCX1388
CAS No.:506-26-3
- Linoleic acid sodium salt
Catalog No.:BCX1387
CAS No.:822-17-3
- Methyl Eicosapentaenoate
Catalog No.:BCX1386
CAS No.:2734-47-6
- Eicosapentaenoic acid ethyl ester
Catalog No.:BCX1385
CAS No.:86227-47-6
- Docosahexaenoic acid methyl ester
Catalog No.:BCX1384
CAS No.:2566-90-7
- Docosahexaenoic acid ethyl ester
Catalog No.:BCX1383
CAS No.:84494-72-4
- Ethyl oleate
Catalog No.:BCX1382
CAS No.:111-62-6
- Elaidic acid
Catalog No.:BCX1395
CAS No.:112-79-8
- 7-Hydroxyisoflavone
Catalog No.:BCX1396
CAS No.:13057-72-2
- 7α-Hydroxysarsasapogenin
Catalog No.:BCX1397
CAS No.:220832-70-2
- Tapinarof
Catalog No.:BCX1398
CAS No.:79338-84-4
- Senecionine acetate
Catalog No.:BCX1399
CAS No.:126642-77-1
- 4,3',5'-Trihydroxyresveratrol
Catalog No.:BCX1400
CAS No.:637776-83-1
- 7β-Hydroxyganoderenic acid F
Catalog No.:BCX1401
CAS No.:1245946-62-6
- 4-Methoxy-2-[(6-O-β-D-xylopyranosyl-β-D-glucopyranosyl)oxy]benzaldehyde
Catalog No.:BCX1402
CAS No.:140484-68-0
- Gylongiposide I
Catalog No.:BCX1403
CAS No.:206876-12-2
- 2'-Deoxyguanosine monohydrate
Catalog No.:BCX1404
CAS No.:312693-72-4
- Emetine hydrobromide
Catalog No.:BCX1405
CAS No.:52714-87-1
- 1β-Methoxydiversifolin 3-O-methyl ether
Catalog No.:BCX1406
CAS No.:194474-71-0
Natural sapogenins as potential inhibitors of aquaporins for targeted cancer therapy: computational insights into binding and inhibition mechanism.[Pubmed:38174738]
J Biomol Struct Dyn. 2024 Jan 4:1-22.
Aquaporins (AQPs) are membrane proteins that facilitate the transport of water and other small molecules across biological membranes. AQPs are involved in various physiological processes and pathological conditions, including cancer, making them as potential targets for anticancer therapy. However, the development of selective and effective inhibitors of AQPs remains a challenge. In this study, we explored the possibility of using natural sapogenins, a class of plant-derived aglycones of saponins with diverse biological activities, as potential inhibitors of AQPs. We performed molecular docking, dynamics simulation and binding energy calculation to investigate the binding and inhibition mechanism of 19 sapogenins against 13 AQPs (AQP0-AQP13) that are overexpressed in various cancers. Our results showed that out of 19 sapogenins, 8 (Diosgenin, Gitogenin, Tigogenin, Ruscogenin, Yamogenin, Hecogenin, Sarsasapogenin and Smilagenin) exhibited acceptable drug-like characteristics. These sapogenin also exhibited favourable binding affinities in the range of -7.6 to -13.4 kcal/mol, and interactions within the AQP binding sites. Furthermore, MD simulations provided insights into stability and dynamics of the sapogenin-AQP complexes. Most of the fluctuations in binding pocket were observed for AQP0-Gitogenin and AQP4-Diosgenin. However, remaining protein-ligand complex showed stable root mean square deviation (RMSD) plots, strong hydrogen bonding interactions, stable solvent-accessible surface area (SASA) values and minimum distance to the receptor. These observations suggest that natural sapogenin hold promise as novel inhibitors of AQPs, offering a basis for the development of innovative therapeutic agents for cancer treatment. However, further validation of the identified compounds through experiments is essential for translating these findings into therapeutic applications.Communicated by Ramaswamy H. Sarma.
Study on the neuroprotective effect of Zhimu-Huangbo extract on mitochondrial dysfunction in HT22 cells induced by D-galactose by promoting mitochondrial autophagy.[Pubmed:37567426]
J Ethnopharmacol. 2024 Jan 10;318(Pt B):117012.
ETHNOPHARMACOLOGICAL RELEVANCE: Zhimu-Huangbo (ZB) herb pair is a common prescription drug used by physicians of all dynasties, and has significant neuroprotective effect, such as the ZB can significantly promote neuronal cell regeneration, repair neuronal damage, and improve cognitive disorders. However, its ingredients are urgently needed to be identified and mechanisms is remained unclear. AIM OF THE STUDY: Using ultra performance liquid chromatography-quadrupole-time of flight-mass spectrometry (UPLC-Q-TOF-MS), the study of neuroprotective mechanism of Zhimu-Huangbo extract (ZBE) is investigated, and the network pharmacology technology and experimental validation is also performed. MATERIAL AND METHODS: Firstly, UPLC-Q-TOF-MS technology was used to characterize the chemical components contained in the ZBE. After that, the TCMSP database and the Swiss Target Prediction method were used to search for potential target genes for ZBE compounds. At the same time, the OMIM and GeneCards disease databases were used to search for Alzheimer's disease (AD) targets and expanded with the GEO database. Then, GO and KEGG enrichment analysis was performed using OECloud tools. Subsequently, the potential mechanism of ZBE therapeutic AD predicted by network pharmacological analysis was experimentally studied and verified in vitro. RESULTS: In the UPLC-Q-TOF-MS analysis of the ZBE, a total of 39 compounds were characterized including Neomangiferin, Oxyberberine, Timosaponin D, Berberine, Timosaponin A-III, Anemarsaponin E, Timosaponin A-I, Smilagenin and so on. A total of 831 potential targets and 13995 AD-related target genes were screened. A further analysis revealed the number of common targets between ZBE and AD is 698. Through GO and KEGG enrichment analysis, we found that ZBE's anti-AD targets were significantly enriched in autophagy and mitochondrial autophagy related pathways. The results of cell experiments also confirmed that ZBE can promote mitochondrial autophagy induced by D-galactose (D-gal) HT22 cells through the PTEN-induced kinase 1/Parkin (PINK1/Parkin) pathway. CONCLUSION: ZBE can promote autophagy of mitochondria and play a protective role on damaged neurons.
Smilagenin induces expression and epigenetic remodeling of BDNF in alzheimer's disease.[Pubmed:37499345]
Phytomedicine. 2023 Sep;118:154956.
BACKGROUND: Smilagenin (SMI) is a lipid-soluble steroidal sapogenin, extracted from traditional Chinses medicinal herbs Radix Asparagi, which is extracted from the dry root of Asparagus cochinchinensis (Lour.) Merr. We previously found that SMI significantly increased brain-derived neurotrophic factor (BDNF) expression in Abeta-intoxicated SH-SY5Y cells. METHODS: In this study, we performed behavioral tests to analyze cognitive function of WT and APP/PS1 mice treated with or without SMI, and found that SMI could significantly improve the learning and memory ability of APP/PS1 mice. Moreover, immunofluorescence and ELISA results showed that SMI pretreatment could effectively reduce the deposition of beta-amyloid plaques in the cortex and hippocampus of APP/PS1 mice (26 mg/kg/day for 60 days) and inhibit the secretion of Abeta1-42 in N2a/APPswe cells (10 muM concentration for 24 hours). RESULTS: Mechanistically, SMI enhanced BDNF mRNA expression, elevated the global level of H3AC and H4AC, and increased the expression of P300 in AD models. Furthermore, chromatin immunoprecipitation results showed that SMI could increase the levels of H3AC and H4AC at the promoter of BDNF promoter Ⅱ and Ⅳ, indicating that SMI epigenetically regulates BDNF expression through HAT enhancement. To further verify the critical role of P300 by which SMI upregulated histone acetylation in BDNF, AD mice were treated with SMI and C646 simultaneously. Behavioral experiments showed that the improvement effects of SMI on cognitive impairment were abolished after P300 inhibition in APP/PS1 mice. CONCLUSIONS: Our research for the first time demonstrated that SMI showed neuroprotective effects by increasing the expression of P300 protein, thus upregulating histone acetylation levels in the promoter region of BDNF and promoting its transcription. Our findings provide an important theoretical basis for the treatment of Alzheimer's disease with SMI extracted from Asparagus cochinchinensis (Lour.) Merr.
Exploration at the network pharmacology level of possible targeting mechanisms of Smilacis Glabrae Rhixoma for the treatment of osteoporosis.[Pubmed:37140318]
Eur Rev Med Pharmacol Sci. 2023 Apr;27(8):3681-3698.
OBJECTIVE: The aim of this study was to evaluate the therapeutic effect of Smilacis Glabrae Rhixoma (SGR) on osteoporosis at the level of network pharmacology, and to find new targets and mechanisms of SGR in the treatment of osteoporosis, to better find new drugs and their clinical applications. MATERIALS AND METHODS: In the original network pharmacology mode, we used an improved mode, such as screening the ingredients and targets of SGR through tools such as GEO database, Autodock Vina, and GROMACS. Through molecular docking, we conducted further screening for the targets acting on the effective ingredients of SGR, and finally we performed molecular dynamics simulation and consulted a large amount of related literature for the validation of the results. RESULTS: By screening and validating the data, we finally confirmed that there were mainly 10 active ingredients in SGR, which were isoeruboside b, Smilagenin, diosgenin, stigmasterol, beta-sitosterol, sodium taurocholate, sitogluside, 4,7-dihydroxy-5-methoxy-6-methyl-8-formyl-flavan, simiglaside B, and simiglaside E, and mainly acted on eleven targets. These targets mainly exert therapeutic effects on osteoporosis by regulating 20 signaling pathways including Th17 cell differentiation, HIF-1 signaling pathway, apoptosis, inflammatory bowel disease, and osteoclast differentiation. CONCLUSIONS: Our study successfully explains the effective mechanism by which SGR ameliorates osteoporosis while predicting the potential targets NFKB1 and CTSK of SGR for the treatment of osteoporosis, which provides a novel basis for investigating the mechanism of action of new Traditional Chinese medicines (TCMs) at the network pharmacology level and a great support for subsequent studies on osteoporosis.


