GuaijaverinCAS# 22255-13-6 |
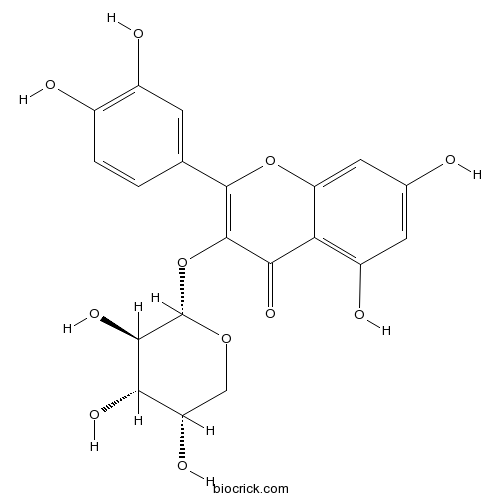
- Quercetin 3-O-beta-D-xylopyranoside
Catalog No.:BCN2851
CAS No.:549-32-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 22255-13-6 | SDF | Download SDF |
| PubChem ID | 5481224 | Appearance | Yellow powder |
| Formula | C20H18O11 | M.Wt | 434.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Feniculin; Foeniculin; Guajaverin; 3,3',4',5,7-Pentahydroxyflavone 3-O-α-L-arabinopyranoside; Quercetin 3-O-α-L-arabinopyranoside | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2S,3R,4S,5S)-3,4,5-trihydroxyoxan-2-yl]oxychromen-4-one | ||
| SMILES | C1C(C(C(C(O1)OC2=C(OC3=CC(=CC(=C3C2=O)O)O)C4=CC(=C(C=C4)O)O)O)O)O | ||
| Standard InChIKey | PZZRDJXEMZMZFD-IEGSVRCHSA-N | ||
| Standard InChI | InChI=1S/C20H18O11/c21-8-4-11(24)14-13(5-8)30-18(7-1-2-9(22)10(23)3-7)19(16(14)27)31-20-17(28)15(26)12(25)6-29-20/h1-5,12,15,17,20-26,28H,6H2/t12-,15-,17+,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Guaijaverin is a urease inhibitor, it (IC(50)=0.18 microM) shows an inhibitory effect on rat lens aldose reductase. Guaijaverin has antioxidant, hypoglycemic activity and inhibitory capacity against free fatty acid release.It demonstrates high potential antiplaque agent by inhibiting the growth of the Strep. Mutans. |
| Targets | GLUT | Antifection |
| In vitro | Guaijaverin -- a plant flavonoid as potential antiplaque agent against Streptococcus mutans.[Pubmed: 16882158]J Appl Microbiol. 2006 Aug;101(2):487-95.The aim of the present study was to investigate the anti-Streptococcus mutans activity and the in vitro effects of subminimal inhibitory concentrations of Guaijaverin isolated from Psidium guajava Linn. on cariogenic properties of Strep. mutans.
Hypoglycemic Activity of Avicularin and Guaijaverin in Guava Leaves.[Reference: WebLink]Food Science, 2016, 37(7):168-74.To study the hypoglycemic activity of avicularin and Guaijaverin in guava leaves. |
| Kinase Assay | The methanolic extracts of several natural medicines and medicinal foodstuffs were found to show an inhibitory effect on rat lens aldose reductase. In most cases, flavonoids were isolated as the active constituents by bioassay-guided separation, and among[Pubmed: 12045333]Inhibitory activity against urease of quercetin glycosides isolated from Allium cepa and Psidium guajava.[Pubmed: 20378972]Biosci Biotechnol Biochem. 2010;74(4):878-80.Methanolic extracts of edible plants and seaweeds were tested for their inhibitory activity against Jack bean urease. Chem. Pharm. Bull. (Tokyo). 2002, 50(6): 788-95.The methanolic extracts of several natural medicines and medicinal foodstuffs were found to show an inhibitory effect on rat lens aldose reductase. |
| Structure Identification | Spectrochim Acta A Mol Biomol Spectrosc. 2012 Nov;97:449-55.Exploring the binding mechanism of Guaijaverin to human serum albumin: fluorescence spectroscopy and computational approach.[Pubmed: 22820048]The Guaijaverin (Gua) is a polyphenolic substance which exhibits some pharmacological activities such as antibacterial and antioxidant activities. |

Guaijaverin Dilution Calculator

Guaijaverin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.302 mL | 11.5101 mL | 23.0203 mL | 46.0405 mL | 57.5506 mL |
| 5 mM | 0.4604 mL | 2.302 mL | 4.6041 mL | 9.2081 mL | 11.5101 mL |
| 10 mM | 0.2302 mL | 1.151 mL | 2.302 mL | 4.6041 mL | 5.7551 mL |
| 50 mM | 0.046 mL | 0.2302 mL | 0.4604 mL | 0.9208 mL | 1.151 mL |
| 100 mM | 0.023 mL | 0.1151 mL | 0.2302 mL | 0.4604 mL | 0.5755 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- alpha-Amyrin palmitate
Catalog No.:BCN5055
CAS No.:22255-10-3
- Methyl 6-hydroxyangolensate
Catalog No.:BCN5054
CAS No.:22255-07-8
- Ipratropium Bromide
Catalog No.:BCC3795
CAS No.:22254-24-6
- 7-Amino-3-methyl-3-cephem-4-carboxylic acid
Catalog No.:BCC8776
CAS No.:22252-43-3
- Fern-7-en-19-one
Catalog No.:BCN6443
CAS No.:222294-61-3
- Thalrugosaminine
Catalog No.:BCN7745
CAS No.:22226-73-9
- Cucurbitacin I
Catalog No.:BCC2439
CAS No.:2222-07-3
- Dehydroglaucine
Catalog No.:BCN2548
CAS No.:22212-26-6
- Dihydroseselin
Catalog No.:BCN8258
CAS No.:2221-66-1
- Naproxen
Catalog No.:BCC9091
CAS No.:22204-53-1
- Pyrantel Pamoate
Catalog No.:BCC4958
CAS No.:22204-24-6
- Lyn peptide inhibitor
Catalog No.:BCC5895
CAS No.:222018-18-0
- Trimethoxystilbene
Catalog No.:BCN6762
CAS No.:22255-22-7
- Lucidin 3-O-glucoside
Catalog No.:BCN8249
CAS No.:22255-29-4
- Loganic acid
Catalog No.:BCN5057
CAS No.:22255-40-9
- Adoprazine
Catalog No.:BCC1329
CAS No.:222551-17-9
- Tempol
Catalog No.:BCC4862
CAS No.:2226-96-2
- Bromocriptine mesylate
Catalog No.:BCC6642
CAS No.:22260-51-1
- Antidesmone
Catalog No.:BCN5058
CAS No.:222629-77-8
- ACBC
Catalog No.:BCC6584
CAS No.:22264-50-2
- Chysin A
Catalog No.:BCN2020
CAS No.:22269-11-0
- SZL P1-41
Catalog No.:BCC8004
CAS No.:222716-34-9
- Noladin ether
Catalog No.:BCC5756
CAS No.:222723-55-9
- Finasteride acetate
Catalog No.:BCC4204
CAS No.:222989-99-3
Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity.[Pubmed:12045333]
Chem Pharm Bull (Tokyo). 2002 Jun;50(6):788-95.
The methanolic extracts of several natural medicines and medicinal foodstuffs were found to show an inhibitory effect on rat lens aldose reductase. In most cases, flavonoids were isolated as the active constituents by bioassay-guided separation, and among them, quercitrin (IC(50)=0.15 microM), Guaijaverin (0.18 microM), and desmanthin-1 (0.082 microM) exhibited potent inhibitory activity. Desmanthin-1 showed the most potent activity, which was equivalent to that of a commercial synthetic aldose reductase inhibitor, epalrestat (0.072 microM). In order to clarify the structural requirements of flavonoids for aldose reductase inhibitory activity, various flavonoids and related compounds were examined. The results suggested the following structural requirements of flavonoid: 1) the flavones and flavonols having the 7-hydroxyl and/or catechol moiety at the B ring (the 3',4'-dihydroxyl moiety) exhibit the strong activity; 2) the 5-hydroxyl moiety does not affect the activity; 3) the 3-hydroxyl and 7-O-glucosyl moieties reduce the activity; 4) the 2-3 double bond enhances the activity; 5) the flavones and flavonols having the catechol moiety at the B ring exhibit stronger activity than those having the pyrogallol moiety (the 3',4',5'-trihydroxyl moiety).
Guaijaverin -- a plant flavonoid as potential antiplaque agent against Streptococcus mutans.[Pubmed:16882158]
J Appl Microbiol. 2006 Aug;101(2):487-95.
AIMS: The aim of the present study was to investigate the anti-Streptococcus mutans activity and the in vitro effects of subminimal inhibitory concentrations of Guaijaverin isolated from Psidium guajava Linn. on cariogenic properties of Strep. mutans. METHODS AND RESULTS: Bioautography-directed chromatographic fractionation, yield biologically active compound, quercetin-3-O-alpha-l-arabinopyranoside (Guaijaverin), from crude methanol extract of P. guajava. Growth-inhibitory activity of the compound against Strep. mutans of both clinical and type strain cultures was evaluated. The anti-Strep. mutans activity of the Guaijaverin was found to be bacteriostatic, both heat and acid stable and alkali labile with the minimum inhibitory concentration (MIC) of 4 mg ml(-1) for MTCC 1943 and 2 mg ml(-1) for CLSM 001. The sub-MIC concentrations (0.0078-2 mg ml(-1)) of the Guaijaverin were evaluated for its cariogenic properties such as acid production, cell-surface hydrophobicity, sucrose-dependent adherence to glass surface and sucrose-induced aggregation of Strep. mutans. CONCLUSIONS: The active flavonoid compound, quercetin-3-O-alpha-l-arabinopyranoside (Guaijaverin) demonstrated high potential antiplaque agent by inhibiting the growth of the Strep. mutans. SIGNIFICANCE AND IMPACT OF THE STUDY: This study demonstrated the new growth-inhibitory compound Guaijaverin against Strep. mutans and led to the acceptance of traditional medicine and natural products as an alternative form of health care.
Inhibitory activity against urease of quercetin glycosides isolated from Allium cepa and Psidium guajava.[Pubmed:20378972]
Biosci Biotechnol Biochem. 2010;74(4):878-80.
Methanolic extracts of edible plants and seaweeds were tested for their inhibitory activity against Jack bean urease. Quercetin-4'-O-beta-D-glucopyranoside was isolated from Allium cepa as a urease inhibitor with an IC(50) value of 190 microM-. Quercetin and two quercetin glycosides, avicularin and Guaijaverin, were isolated from Psidium guajava as urease inhibitors with respective IC(50) values of 80 microM-, 140 microM-, and 120 microM-.
Exploring the binding mechanism of Guaijaverin to human serum albumin: fluorescence spectroscopy and computational approach.[Pubmed:22820048]
Spectrochim Acta A Mol Biomol Spectrosc. 2012 Nov;97:449-55.
The Guaijaverin (Gua) is a polyphenolic substance which exhibits some pharmacological activities such as antibacterial and antioxidant activities. Here we have investigated the binding of Gua with human serum albumin (HSA) at physiological pH 7.0. In this study, the fluorescence spectroscopy, ab initio and molecular modeling calculations were applied. The Stern-Volmer quenching constant (K(SV)) and its modified form (K(a)) were calculated at 298, 303 and 308 K, with the corresponding thermodynamic parameters DeltaH, DeltaG and DeltaS as well. The fluorescence quenching method was used to determine the number of binding sites (n) and binding constants (K(b)) values at 298, 303 and 308 K. The distance between donor (HSA) and acceptor (Gua) was estimated according to fluorescence resonance energy transfer. The geometry optimization of Gua was performed in its ground state by using ab initio DFT/B3LYP functional with a 6-31G(d,p) basis set used in calculations. Molecular modeling calculation indicated that the Gua is located within the hydrophobic pocket of the subdomain IIA of HSA. The theoretical results obtained by molecular modeling were corroborated by fluorescence spectroscopy data.


