H-Phe-NH2CAS# 5241-58-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5241-58-7 | SDF | Download SDF |
| PubChem ID | 445694 | Appearance | Powder |
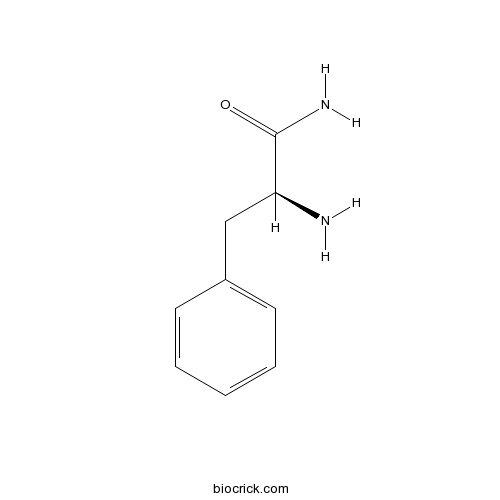
| Formula | C9H12N2O | M.Wt | 164.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-amino-3-phenylpropanamide | ||
| SMILES | C1=CC=C(C=C1)CC(C(=O)N)N | ||
| Standard InChIKey | OBSIQMZKFXFYLV-QMMMGPOBSA-N | ||
| Standard InChI | InChI=1S/C9H12N2O/c10-8(9(11)12)6-7-4-2-1-3-5-7/h1-5,8H,6,10H2,(H2,11,12)/t8-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

H-Phe-NH2 Dilution Calculator

H-Phe-NH2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0901 mL | 30.4507 mL | 60.9013 mL | 121.8027 mL | 152.2533 mL |
| 5 mM | 1.218 mL | 6.0901 mL | 12.1803 mL | 24.3605 mL | 30.4507 mL |
| 10 mM | 0.609 mL | 3.0451 mL | 6.0901 mL | 12.1803 mL | 15.2253 mL |
| 50 mM | 0.1218 mL | 0.609 mL | 1.218 mL | 2.4361 mL | 3.0451 mL |
| 100 mM | 0.0609 mL | 0.3045 mL | 0.609 mL | 1.218 mL | 1.5225 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H-Phe-NH2
- (R)-DPN
Catalog No.:BCC7939
CAS No.:524047-78-7
- Noricaritin
Catalog No.:BCN5353
CAS No.:5240-95-9
- 2-APB
Catalog No.:BCC6978
CAS No.:524-95-8
- Fraxin
Catalog No.:BCN1237
CAS No.:524-30-1
- Dauricine
Catalog No.:BCN4977
CAS No.:524-17-4
- gamma-Fagarine
Catalog No.:BCN5673
CAS No.:524-15-2
- Wedelolactone
Catalog No.:BCN5672
CAS No.:524-12-9
- Epistephamiersine
Catalog No.:BCN5671
CAS No.:52389-15-8
- Erysotramidine
Catalog No.:BCN5670
CAS No.:52358-58-4
- 2-Benzoyl-1,3,4,4a,5,8a-hexahydro-6(2H)-isoquinolinone
Catalog No.:BCC8559
CAS No.:52346-14-2
- Alnusone
Catalog No.:BCN8108
CAS No.:52330-11-7
- 6alpha-Chloro-5beta-hydroxywithaferin A
Catalog No.:BCN8007
CAS No.:52329-20-1
- Boc-D-Trp-OH
Catalog No.:BCC3457
CAS No.:5241-64-5
- Boc-D-Met-OH
Catalog No.:BCC3426
CAS No.:5241-66-7
- Cochlearine
Catalog No.:BCN1929
CAS No.:52418-07-2
- Oxeladin Citrate
Catalog No.:BCC3831
CAS No.:52432-72-1
- Isobutylshikonin
Catalog No.:BCN3005
CAS No.:52438-12-7
- 1-Cinnamoylpyrrolidine
Catalog No.:BCN4086
CAS No.:52438-21-8
- ERB 041
Catalog No.:BCC7903
CAS No.:524684-52-4
- Vandetanib hydrochloride
Catalog No.:BCC2028
CAS No.:524722-52-9
- Isokurarinone
Catalog No.:BCN2890
CAS No.:52483-02-0
- 5-(Z-heptadec-8-enyl) resorcinol
Catalog No.:BCN5674
CAS No.:52483-19-9
- D-Tryptophanol
Catalog No.:BCC2699
CAS No.:52485-52-6
- Peonidin-3-O-arabinoside chloride
Catalog No.:BCN3029
CAS No.:524943-91-7
Total enzymatic synthesis of cholecystokinin CCK-5.[Pubmed:15309578]
Amino Acids. 2004 Aug;27(1):101-5.
This paper describes the enzymatic synthesis of the C-terminal fragment H-Gly-Trp-Met-Asp-Phe-NH2 of cholecystokinin. Immobilized enzymes were used for the formation of all peptide bonds except thermolysin. Beginning the synthesis with phenylacetyl (PhAc) glycine carboxamidomethyl ester (OCam) and H-Trp-OMe by using immobilized papain as biocatalyst in buffered ethyl acetate, the dipeptide methyl ester was then coupled directly with Met-OEt.HCl by alpha-chymotrypsin/Celite 545 in a solvent free system. For the 3+2 coupling PhAc-Gly-Trp-Met-OEt had to be converted into its OCam ester. The other fragment H-Asp(OMe)-Phe-NH2 resulted from the coupling of Cbo-Asp(OMe)-OH with H-Phe-NH2.HCl and thermolysin as catalyst, followed by catalytic hydrogenation. Finally PhAc-Gly-Trp-Met-Asp-Phe-NH2 was obtained in a smooth reaction from PhAc-Gly-Trp-Met-OCam and H-Asp(OMe)-Phe-NH2 with alpha-chymotrypsin/Celite 545 in acetonitrile, followed by basic hydrolysis of the beta-methyl ester. The PhAc-group is removed with penicillin G amidase and CCK-5 is obtained in an overall isolated yield of 19.6%.
Enzymatic synthesis of a CCK-4 tripeptide fragment.[Pubmed:12697455]
Di Yi Jun Yi Da Xue Xue Bao. 2003 Apr;23(4):289-92.
OBJECTIVE: To synthesize a tripeptide derivative Phac-Met-Asp(OMe)-Phe -NH2, which is a fragment of the gastrin C-terminal tetrapeptide CCK-4, by enzymatic reaction. METHODS: Three free enzymes, alpha-chymotrypsin, papain and thermolysin from acyl donor Phac-Met-OCam was involved in three steps. The choice of appropriate enzymes and solvents was selected. RESULTS: All enzymatic reactions were obtained in reasonable yields(63%-92%). FAB-MS and FD-MS verified the correct molecular mass of the peptides. CONCLUSION: Studies on the alpha-chymotrypsin catalyzed coupling reaction between Phac-Met-OCam and H-Asp(OMe)2 have focused on the low water content media. By papain catalyzed saponification of Phac-Met-Asp(OMe)2, alpha-methyl ester of aspartic acid is selectively hydrolyzed to retain beta-methyl ester, and Phac-Met-Asp(OMe)-OH and H-Phe-NH2 can be coupled efficiently by thermolysin.


