IsobutylshikoninCAS# 52438-12-7 |
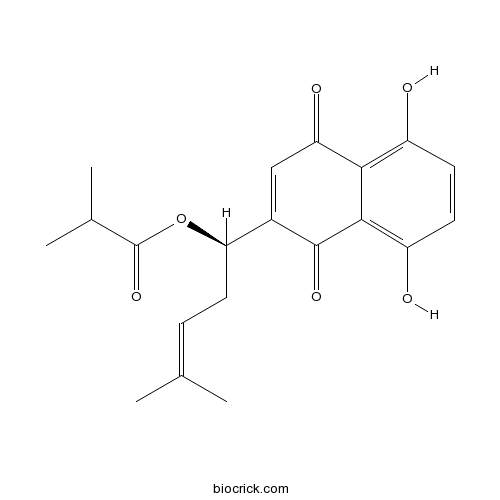
- Isobutyryl alkannin
Catalog No.:BCX1535
CAS No.:87562-78-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 52438-12-7 | SDF | Download SDF |
| PubChem ID | 479500 | Appearance | Powder |
| Formula | C20H22O6 | M.Wt | 358.4 |
| Type of Compound | Quinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1R)-1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] 2-methylpropanoate | ||
| SMILES | CC(C)C(=O)OC(CC=C(C)C)C1=CC(=O)C2=C(C=CC(=C2C1=O)O)O | ||
| Standard InChIKey | BVRYLTBIGIAADD-MRXNPFEDSA-N | ||
| Standard InChI | InChI=1S/C20H22O6/c1-10(2)5-8-16(26-20(25)11(3)4)12-9-15(23)17-13(21)6-7-14(22)18(17)19(12)24/h5-7,9,11,16,21-22H,8H2,1-4H3/t16-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Isobutylshikonin exhibits obvious antioxidant activities , it exerts very good radical scavenging activities toward ABTS+ but shows moderate inhibition of DPPH·. |
| In vitro | Physical stability of shikonin derivatives from the roots of Lithospermum erythrorhizon cultivated in Korea.[Pubmed: 10552776]J Agric Food Chem. 1999 Oct;47(10):4117-20.
Antioxidants from a Chinese medicinal herb – Lithospermum erythrorhizon[Reference: WebLink]Food Chemistry, 2008, 106(1):2-10.Seven compounds, deoxyshikonin (1), β,β-dimethylacrylshikonin (2), Isobutylshikonin (3), shikonin (4), 5,8-dihydroxy-2-(1-methoxy-4-methyl-3-pentenyl)-1,4-naphthalenedione (5), β-sitosterol (6) and a mixture of two caffeic acid esters [7 (7a,7b)] were isolated from Lithospermum erythrorhizon Sieb et. Zucc. and identified by spectroscopic methods.
Among them, compound 5 was isolated from this plant species for the first time.
|
| Structure Identification | Anal Chim Acta. 2006 Sep 1;577(1):26-31.Simultaneous determination of naphthoquinone derivatives in Boraginaceous herbs by high-performance liquid chromatography.[Pubmed: 17723649]
|

Isobutylshikonin Dilution Calculator

Isobutylshikonin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7902 mL | 13.9509 mL | 27.9018 mL | 55.8036 mL | 69.7545 mL |
| 5 mM | 0.558 mL | 2.7902 mL | 5.5804 mL | 11.1607 mL | 13.9509 mL |
| 10 mM | 0.279 mL | 1.3951 mL | 2.7902 mL | 5.5804 mL | 6.9754 mL |
| 50 mM | 0.0558 mL | 0.279 mL | 0.558 mL | 1.1161 mL | 1.3951 mL |
| 100 mM | 0.0279 mL | 0.1395 mL | 0.279 mL | 0.558 mL | 0.6975 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Oxeladin Citrate
Catalog No.:BCC3831
CAS No.:52432-72-1
- Cochlearine
Catalog No.:BCN1929
CAS No.:52418-07-2
- Boc-D-Met-OH
Catalog No.:BCC3426
CAS No.:5241-66-7
- Boc-D-Trp-OH
Catalog No.:BCC3457
CAS No.:5241-64-5
- H-Phe-NH2
Catalog No.:BCC3008
CAS No.:5241-58-7
- (R)-DPN
Catalog No.:BCC7939
CAS No.:524047-78-7
- Noricaritin
Catalog No.:BCN5353
CAS No.:5240-95-9
- 2-APB
Catalog No.:BCC6978
CAS No.:524-95-8
- Fraxin
Catalog No.:BCN1237
CAS No.:524-30-1
- Dauricine
Catalog No.:BCN4977
CAS No.:524-17-4
- gamma-Fagarine
Catalog No.:BCN5673
CAS No.:524-15-2
- Wedelolactone
Catalog No.:BCN5672
CAS No.:524-12-9
- 1-Cinnamoylpyrrolidine
Catalog No.:BCN4086
CAS No.:52438-21-8
- ERB 041
Catalog No.:BCC7903
CAS No.:524684-52-4
- Vandetanib hydrochloride
Catalog No.:BCC2028
CAS No.:524722-52-9
- Isokurarinone
Catalog No.:BCN2890
CAS No.:52483-02-0
- 5-(Z-heptadec-8-enyl) resorcinol
Catalog No.:BCN5674
CAS No.:52483-19-9
- D-Tryptophanol
Catalog No.:BCC2699
CAS No.:52485-52-6
- Peonidin-3-O-arabinoside chloride
Catalog No.:BCN3029
CAS No.:524943-91-7
- PSB 36
Catalog No.:BCC7240
CAS No.:524944-72-7
- Dihydrokainic acid
Catalog No.:BCC6556
CAS No.:52497-36-6
- Fraxidin
Catalog No.:BCN5675
CAS No.:525-21-3
- Harmalol
Catalog No.:BCC8183
CAS No.:525-57-5
- Kinetin
Catalog No.:BCC1679
CAS No.:525-79-1
Physical stability of shikonin derivatives from the roots of Lithospermum erythrorhizon cultivated in Korea.[Pubmed:10552776]
J Agric Food Chem. 1999 Oct;47(10):4117-20.
Five red shikonin pigments, deoxyshikonin, shikonin, acetylshikonin, Isobutylshikonin, and beta-hydroxyisovalerylshikonin, were isolated from the roots of Lithospermum erythrorhizon cultivated in Korea. The purified pigments were red, purple, and blue at acidic, neutral, and alkaline pH values, respectively. Physical stability of the purified pigments against heat and light in an aqueous solution was examined for possible value-added food colorants. The thermal degradation reactions were carried out at pH 3.0 (50 mM glycine buffer) in 50% EtOH/H(2)O. Deoxyshikonin (t(1/2) = 14.6 h, 60 degrees C) and isobutylshikinin (t(1/2) = 19.3 h, 60 degrees C) are relatively less stable than other shikonin derivatives (t(1/2) = 40-50 h, 60 degrees C). Activation energies of thermal degradation of the isolated pigments were calculated. The activation energy of deoxyshikonin was the highest (12.5 kcal mol(-)(1)) and that of beta-hydroxyisovalerylshikonin was the lowest (1.71 kcal mol(-)(1)) value. Light stabilities of the pigments were similar to each other in that the half-life values of photodegradation for 20000 lx light intensity were 4.2-5.1 h.
Simultaneous determination of naphthoquinone derivatives in Boraginaceous herbs by high-performance liquid chromatography.[Pubmed:17723649]
Anal Chim Acta. 2006 Sep 1;577(1):26-31.
A high-performance liquid chromatographic method using diode-array detection (HPLC-DAD) has been developed for the simultaneous quantification of eight naphthoquinone derivatives namely shikonin, acetylshikonin, deoxyshikonin, beta-acetoxyisovalerylshikonin, Isobutylshikonin, beta,beta-dimethylacrylshikonin, 2-methyl-n-butyrylshikonin and isovalerylshikonin in nine species of the Boraginaceae family. These species, coming from different areas of China, are all used as interchangeable sourcing plants for the Chinese Materia Medica known as "Zicao", and are Arnebia euchroma (Royle) Johnston., A. guttata Bunge, Lithospermum erythrorhizon Sieb. et Zucc., Onosma paniculatum Bur. et Franch., O. exsertum Hemsl., O. confertum W.W. Smith, O. hookerii Clarke var. longiflorum Duthie, O. hookerii Clarke and O. waltonii Duthic. Quantification of the eight naphthoquinones in all the Zicao samples are reported and compared with each other. Furthermore, two positional isomers, 2-methyl-n-butyrylshikonin and isovalerylshikonin, were successfully separated and quantified for the first time in the present study. The results showed that, besides the three officially used species (namely, A. euchroma, A. guttata and L. erythrorhizon) that were listed in Chinese pharmacopoeia as interchangeable sourcing plants for Zicao, other six species of Onosma used by native peoples in Tibet and Yunnan Province also contain various types and considerable amounts of naphthoquinones and that O. waltonii contains the most. Therefore, these species of Onosma could be developed as new sources of naphthoquinones. The entire analytical procedure is reproducible and suitable for the quantification of naphthoquinones in all related Boraginaceous plants for quality assessment purposes.


