INCA-6Selective calcineurin-NFAT signaling inhibitor CAS# 3519-82-2 |
- NFAT Inhibitor
Catalog No.:BCC2463
CAS No.:249537-73-3
Quality Control & MSDS
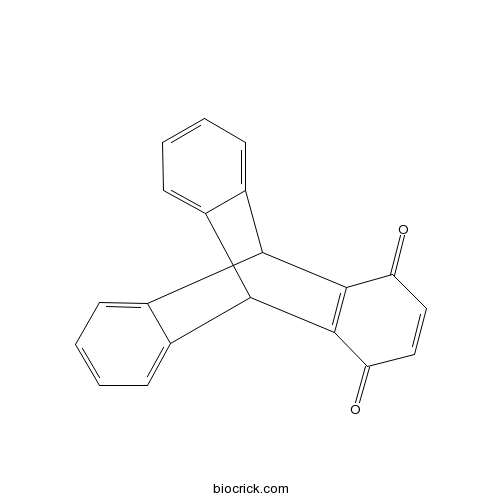
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3519-82-2 | SDF | Download SDF |
| PubChem ID | 230748 | Appearance | Powder |
| Formula | C20H12O2 | M.Wt | 284.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 20 mM in DMSO | ||
| SMILES | C1=CC=C2C3C4=CC=CC=C4C(C2=C1)C5=C3C(=O)C=CC5=O | ||
| Standard InChIKey | GCHPUOHXXCNSQL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H12O2/c21-15-9-10-16(22)20-18-12-6-2-1-5-11(12)17(19(15)20)13-7-3-4-8-14(13)18/h1-10,17-18H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of interaction between calcineurin and its substrate nuclear factor of activated T cells (NFAT); blocks at the substrate recognition site but not at the catalytic site (Kd = 0.8 mM). Inhibits NFAT dephosphorylation and nuclear import. Also prevents induction of cytokine mRNAs that are downstream targets of NFAT. | |||||

INCA-6 Dilution Calculator

INCA-6 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5173 mL | 17.5864 mL | 35.1729 mL | 70.3457 mL | 87.9322 mL |
| 5 mM | 0.7035 mL | 3.5173 mL | 7.0346 mL | 14.0691 mL | 17.5864 mL |
| 10 mM | 0.3517 mL | 1.7586 mL | 3.5173 mL | 7.0346 mL | 8.7932 mL |
| 50 mM | 0.0703 mL | 0.3517 mL | 0.7035 mL | 1.4069 mL | 1.7586 mL |
| 100 mM | 0.0352 mL | 0.1759 mL | 0.3517 mL | 0.7035 mL | 0.8793 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
INCA-6 is a selective inhibitor of transcription factor NFAT with Kd value of 0.8 mM in vitro[1].
NFAT (Nuclear factor of activated T-cells) is a general name which is applied to a family of transcription factors that are important in immune response. They are expressed in most immune system cells. Calcium signaling plays an important role in NFAT activation. Calmodulin activates calcineurin (CN) which is a serine/threonine phosphatase, then dephosphorylates the amino termini of NFAT proteins resulting a conformational change. NFAT will transport into nuclear. NFAT proteins will cooperate with other nuclear resident transcription factors due to that they have weak DNA-binding capacity. NFAT proteins have weak DNA-binding capacity NFAT transcription factors are specifically implicated in the process of cell motility and are related to breast cancer.[2]
INCA-6 treatment significant blocked translocation of nuclear factor of activated T-cells c1. INCA-6 effectively blocked the stimulation of vascular endothelial growth factor stimulation at 1.0 μM. INCA-6 significantly tube formation at 1.0μM in human retinal microvascular endothelial cells[1]. INCA-6 at 50 μM inhibited ATP-induced activation of NFATc1 in MG-5 cells.[3] In BV-2 cells, INCA-6 at 10
μM significantly inhibited ATP-induced CXCL2 expression.[4] INCA-6 reversed the proliferative effect of E2.[5]
References:
[1]. Bretz CA, Savage S, Capozzi M, Penn JS: The role of the NFAT signaling pathway in retinal neovascularization. Invest Ophthalmol Vis Sci 2013, 54(10):7020-7027.
[2]. Crabtree GR, Olson EN: NFAT signaling: choreographing the social lives of cells. Cell 2002, 109 Suppl:S67-79.
[3]. Kataoka A, Tozaki-Saitoh H, Koga Y, Tsuda M, Inoue K: Activation of P2X7 receptors induces CCL3 production in microglial cells through transcription factor NFAT. J Neurochem 2009, 108(1):115-125.
[4]. Shiratori M, Tozaki-Saitoh H, Yoshitake M, Tsuda M, Inoue K: P2X7 receptor activation induces CXCL2 production in microglia through NFAT and PKC/MAPK pathways. J Neurochem 2010, 114(3):810-819.
[5]. Wong CK, So WY, Law SK, Leung FP, Yau KL, Yao X, Huang Y, Li X, Tsang SY: Estrogen controls embryonic stem cell proliferation via store-operated calcium entry and the nuclear factor of activated T-cells (NFAT). J Cell Physiol 2012, 227(6):2519-2530.
- Isopropylidenylacetyl-marmesin
Catalog No.:BCN6792
CAS No.:35178-20-2
- 5-Nonadecylresorcinol
Catalog No.:BCN7629
CAS No.:35176-46-6
- Deacylmetaplexigenin
Catalog No.:BCC8163
CAS No.:3513-04-0
- Blasticidin S HCl
Catalog No.:BCC5565
CAS No.:3513-03-9
- GNTI dihydrochloride
Catalog No.:BCC7003
CAS No.:351183-88-5
- Adynerin
Catalog No.:BCN4643
CAS No.:35109-93-4
- H-D-His-OH
Catalog No.:BCC2959
CAS No.:351-50-8
- Dendocarbin A
Catalog No.:BCN5287
CAS No.:350986-74-2
- Isodemethylwedelolacton
Catalog No.:BCN2766
CAS No.:350681-33-3
- 4-Epi-curcumenol
Catalog No.:BCN3523
CAS No.:350602-21-0
- Obscuraminol B
Catalog No.:BCN1766
CAS No.:350485-82-4
- Obscuraminol F
Catalog No.:BCN1768
CAS No.:350485-01-7
- D-Tetrahydropalmatine
Catalog No.:BCN2334
CAS No.:3520-14-7
- JC-1
Catalog No.:BCC1669
CAS No.:3520-43-2
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- 4,5-Dimethoxy-1-cyanobenzocyclobutane
Catalog No.:BCC8665
CAS No.:35202-54-1
- Ipriflavone (Osteofix)
Catalog No.:BCC5323
CAS No.:35212-22-7
- Neobyakangelicol
Catalog No.:BCN5288
CAS No.:35214-82-5
- Alloisoimperatorin
Catalog No.:BCN6789
CAS No.:35214-83-6
- 4-(Bromomethyl)-7-methoxy coumarin
Catalog No.:BCC9202
CAS No.:35231-44-8
- Benzoin methyl ether
Catalog No.:BCC8857
CAS No.:3524-62-7
- 9-Methoxy-alpha-lapachone
Catalog No.:BCN5289
CAS No.:35241-80-6
- Delafloxacin meglumine
Catalog No.:BCC1523
CAS No.:352458-37-8
- Caesalmin B
Catalog No.:BCN7252
CAS No.:352658-23-2
NFAT isoforms play distinct roles in TNFalpha-induced retinal leukostasis.[Pubmed:26527057]
Sci Rep. 2015 Nov 3;5:14963.
The objective of this study was to determine the role of individual NFAT isoforms in TNFalpha-induced retinal leukostasis. To this end, human retinal microvascular endothelial cells (HRMEC) transfected with siRNA targeting individual NFAT isoforms were treated with TNFalpha, and qRT-PCR was used to examine the contribution of each isoform to the TNFalpha-induced upregulation of leukocyte adhesion proteins. This showed that NFATc1 siRNA increased ICAM1 expression, NFATc2 siRNA reduced CX3CL1, VCAM1, SELE, and ICAM1 expression, NFATc3 siRNA increased CX3CL1 and SELE expression, and NFATc4 siRNA reduced SELE expression. Transfected HRMEC monolayers were also treated with TNFalpha and assayed using a parallel plate flow chamber, and both NFATc2 and NFATc4 knockdown reduced TNFalpha-induced cell adhesion. The effect of isoform-specific knockdown on TNFalpha-induced cytokine production was also measured using protein ELISAs and conditioned cell culture medium, and showed that NFATc4 siRNA reduced CXCL10, CXCL11, and MCP-1 protein levels. Lastly, the CN/NFAT-signaling inhibitor INCA-6 was shown to reduce TNFalpha-induced retinal leukostasis in vivo. Together, these studies show a clear role for NFAT-signaling in TNFalpha-induced retinal leukostasis, and identify NFATc2 and NFATc4 as potentially valuable therapeutic targets for treating retinopathies in which TNFalpha plays a pathogenic role.
Calcineurin inhibitors regulate fibroblast growth factor 23 (FGF23) synthesis.[Pubmed:28761977]
Naunyn Schmiedebergs Arch Pharmacol. 2017 Nov;390(11):1117-1123.
Fibroblast growth factor 23 (FGF23) inhibits renal phosphate reabsorption and calcitriol formation, effects depending on Klotho as a co-receptor for FGF23. In addition, FGF23/Klotho strongly influences aging and the onset of age-associated diseases. The synthesis of FGF23 by bone cells is induced by store-operated Ca(2+) entry (SOCE) through Orai1 in UMR106 osteoblast-like cells. Ca(2+) entry activates the phosphatase calcineurin in many cell types which dephosphorylates nuclear factor of activated T cells (NFAT) thereby stimulating its transcriptional activity. Here, we explored whether calcineurin-NFAT signaling impacts on FGF23 production. Fgf23 transcripts were determined by qRT-PCR and FGF23 protein by ELISA. Calcineurin as well as NFAT expression were quantified by RT-PCR in UMR106 cells. UMR106 cells expressed calcineurin subunits Ppp3r1, Ppp3ca, Ppp3cb, and Ppp3cc as well as NFATc1, NFATc3, and NFATc4. Calcineurin inhibitors ciclosporin A (CsA) and tacrolimus (FK-506) decreased Fgf23 gene expression and FGF23 protein production. Moreover, calcineurin-NFAT interaction inhibitor INCA-6 reduced the abundance of Fgf23 transcripts as well as FGF23 protein. Calcineurin-NFAT signaling is a potent regulator of FGF23 formation.
The cardiac calsequestrin gene transcription is modulated at the promoter by NFAT and MEF-2 transcription factors.[Pubmed:28886186]
PLoS One. 2017 Sep 8;12(9):e0184724.
Calsequestrin-2 (CASQ2) is the main Ca2+-binding protein inside the sarcoplasmic reticulum of cardiomyocytes. Previously, we demonstrated that MEF-2 and SRF binding sites within the human CASQ2 gene (hCASQ2) promoter region are functional in neonatal cardiomyocytes. In this work, we investigated if the calcineurin/NFAT pathway regulates hCASQ2 expression in neonatal cardiomyocytes. The inhibition of NFAT dephosphorylation with CsA or INCA-6, reduced both the luciferase activity of hCASQ2 promoter constructs (-3102/+176 bp and -288/+176 bp) and the CASQ2 mRNA levels in neonatal rat cardiomyocytes. Additionally, NFATc1 and NFATc3 over-expressing neonatal cardiomyocytes showed a 2-3-fold increase in luciferase activity of both hCASQ2 promoter constructs, which was prevented by CsA treatment. Site-directed mutagenesis of the -133 bp MEF-2 binding site prevented trans-activation of hCASQ2 promoter constructs induced by NFAT overexpression. Chromatin Immunoprecipitation (ChIP) assays revealed NFAT and MEF-2 enrichment within the -288 bp to +76 bp of the hCASQ2 gene promoter. Besides, a direct interaction between NFAT and MEF-2 proteins was demonstrated by protein co-immunoprecipitation experiments. Taken together, these data demonstrate that NFAT interacts with MEF-2 bound to the -133 bp binding site at the hCASQ2 gene promoter. In conclusion, in this work, we demonstrate that the Ca2+-calcineurin/NFAT pathway modulates the transcription of the hCASQ2 gene in neonatal cardiomyocytes.
Luteolin and apigenin activate the Oct-4/Sox2 signal via NFATc1 in human periodontal ligament cells.[Pubmed:27449921]
Cell Biol Int. 2016 Oct;40(10):1094-106.
Identifying small molecules to activate the Oct-4/Sox2-derived pluripotency network represents a hopeful and safe method to pluripotency without genetic manipulation. Luteolin and apigenin, two major bioactive flavonoids, enhance reprogramming efficiency and increase expression of Oct-4/Sox2/c-Myc, albeit the detailed mechanism regulating pluripotency in dental-derived cells remains unknown. In the present study, to elucidate the effect of luteolin/apigenin on pluripotency of periodontal ligament cells (PDLCs) through interaction with downstream signals, we examined cell cycle, proliferation, apoptosis, expression of Oct-4/Sox2/c-Myc, and multilineage differentiation of PDLCs with luteolin/apigenin treatment. Moreover, we profiled the differentially expressed pluripotency genes by PCR arrays. Our results demonstrated that luteolin/apigenin restrained cell proliferation, increased apoptosis, and arrested PDLCs in G2/M and S phase. Luteolin and apigenin activated expression of Oct-4, Sox2, and c-Myc in a time- and dose-dependent pattern, and repressed lineage-specific differentiation. PCR arrays profiled multiple signals in PDLCs with luteolin/apigenin treatment, among which NFATc1 was the major upregulated gene. Notably, blocking of the NFATc1 signal with INCA-6 significantly decreased mRNA and protein expression of Oct-4, Sox2, and c-Myc in PDLCs with luteolin/apigenin treatment, indicating that NFATc1 may act as an upstream modulator of Oct-4/Sox2 signal. Taken together, this study showed that luteolin and apigenin effectively maintain pluripotency of PDLCs through activation of Oct-4/Sox2 signal via NFATc1.
RNA-Seq reveals a role for NFAT-signaling in human retinal microvascular endothelial cells treated with TNFalpha.[Pubmed:25617622]
PLoS One. 2015 Jan 24;10(1):e0116941.
TNFalpha has been identified as playing an important role in pathologic complications associated with diabetic retinopathy and retinal inflammation, such as retinal leukostasis. However, the transcriptional effects of TNFalpha on retinal microvascular endothelial cells and the different signaling pathways involved are not yet fully understood. In the present study, RNA-seq was used to profile the transcriptome of human retinal microvascular endothelial cells (HRMEC) treated for 4 hours with TNFalpha in the presence or absence of the NFAT-specific inhibitor INCA-6, in order to gain insight into the specific effects of TNFalpha on RMEC and identify any involvement of NFAT signaling. Differential expression analysis revealed that TNFalpha treatment significantly upregulated the expression of 579 genes when compared to vehicle-treated controls, and subsequent pathway analysis revealed a TNFalpha-induced enrichment of transcripts associated with cytokine-cytokine receptor interactions, cell adhesion molecules, and leukocyte transendothelial migration. Differential expression analysis comparing TNFalpha-treated cells to those co-treated with INCA-6 revealed 10 genes whose expression was significantly reduced by the NFAT inhibitor, including those encoding the proteins VCAM1 and CX3CL1 and cytokines CXCL10 and CXCL11. This study identifies the transcriptional effects of TNFalpha on HRMEC, highlighting its involvement in multiple pathways that contribute to retinal leukostasis, and identifying a previously unknown role for NFAT-signaling downstream of TNFalpha.


