Obscuraminol FCAS# 350485-01-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 350485-01-7 | SDF | Download SDF |
| PubChem ID | 101101129 | Appearance | Oil |
| Formula | C16H33NO | M.Wt | 255.44 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
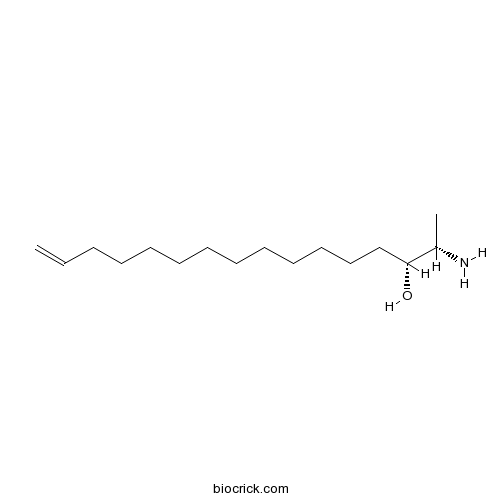
| Chemical Name | (2S,3R)-2-aminohexadec-15-en-3-ol | ||
| SMILES | CC(C(CCCCCCCCCCCC=C)O)N | ||
| Standard InChIKey | BBZHHOGHQKXCRQ-JKSUJKDBSA-N | ||
| Standard InChI | InChI=1S/C16H33NO/c1-3-4-5-6-7-8-9-10-11-12-13-14-16(18)15(2)17/h3,15-16,18H,1,4-14,17H2,2H3/t15-,16+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Tetrahedron, 2001, 57(21):4579-4588.Obscuraminols, new unsaturated amino alcohols from the tunicate Pseudodistoma obscurum: structure and absolute configuration.[Reference: WebLink]
|

Obscuraminol F Dilution Calculator

Obscuraminol F Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9148 mL | 19.5741 mL | 39.1481 mL | 78.2963 mL | 97.8703 mL |
| 5 mM | 0.783 mL | 3.9148 mL | 7.8296 mL | 15.6593 mL | 19.5741 mL |
| 10 mM | 0.3915 mL | 1.9574 mL | 3.9148 mL | 7.8296 mL | 9.787 mL |
| 50 mM | 0.0783 mL | 0.3915 mL | 0.783 mL | 1.5659 mL | 1.9574 mL |
| 100 mM | 0.0391 mL | 0.1957 mL | 0.3915 mL | 0.783 mL | 0.9787 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Obscuraminol E
Catalog No.:BCN1769
CAS No.:350485-00-6
- Obscuraminol D
Catalog No.:BCN1770
CAS No.:350484-99-0
- Obscuraminol C
Catalog No.:BCN1767
CAS No.:350484-95-6
- 2,5-Dihydroxyxanthone
Catalog No.:BCN7573
CAS No.:35040-32-5
- ent-17-Hydroxykaur-15-en-19-oic acid
Catalog No.:BCN4644
CAS No.:35030-38-7
- Brosimacutin G
Catalog No.:BCN3202
CAS No.:350221-50-0
- NHS-Biotin
Catalog No.:BCC3577
CAS No.:35013-72-0
- Cucurbitadienol
Catalog No.:BCN3342
CAS No.:35012-08-9
- 10beta-Hydroxycadina-4,11(13)-dien-12,8beta-olide
Catalog No.:BCN7435
CAS No.:35001-23-1
- 4alpha,6alpha-Dihydroxyeudesm-11(13)-en-12,8beta-olide
Catalog No.:BCN7431
CAS No.:35001-19-5
- Kurarinone
Catalog No.:BCN2985
CAS No.:34981-26-5
- Kuraridine
Catalog No.:BCN2988
CAS No.:34981-25-4
- Obscuraminol B
Catalog No.:BCN1766
CAS No.:350485-82-4
- 4-Epi-curcumenol
Catalog No.:BCN3523
CAS No.:350602-21-0
- Isodemethylwedelolacton
Catalog No.:BCN2766
CAS No.:350681-33-3
- Dendocarbin A
Catalog No.:BCN5287
CAS No.:350986-74-2
- H-D-His-OH
Catalog No.:BCC2959
CAS No.:351-50-8
- Adynerin
Catalog No.:BCN4643
CAS No.:35109-93-4
- GNTI dihydrochloride
Catalog No.:BCC7003
CAS No.:351183-88-5
- Blasticidin S HCl
Catalog No.:BCC5565
CAS No.:3513-03-9
- Deacylmetaplexigenin
Catalog No.:BCC8163
CAS No.:3513-04-0
- 5-Nonadecylresorcinol
Catalog No.:BCN7629
CAS No.:35176-46-6
- Isopropylidenylacetyl-marmesin
Catalog No.:BCN6792
CAS No.:35178-20-2
- INCA-6
Catalog No.:BCC2462
CAS No.:3519-82-2
Testosterone-induced modulation of peroxisomal morphology and peroxisome-related gene expression in brown trout (Salmo trutta f. fario) primary hepatocytes.[Pubmed:29032351]
Aquat Toxicol. 2017 Dec;193:30-39.
Disruption of androgenic signaling has been linked to possible cross-modulation with other hormone-mediated pathways. Therefore, our objective was to explore effects caused by testosterone - T (1, 10 and 50muM) in peroxisomal signaling of brown trout hepatocytes. To study the underlying paths involved, several co-exposure conditions were tested, with flutamide - F (anti-androgen) and ICI 182,780 - ICI (anti-estrogen). Molecular and morphological approaches were both evaluated. Peroxisome proliferator-activated receptor alpha (PPARalpha), catalase and urate oxidase were the selected targets for gene expression analysis. The vitellogenin A gene was also included as a biomarker of estrogenicity. Peroxisome relative volumes were estimated by immunofluorescence, and transmission electron microscopy was used for qualitative morphological control. The single exposures of T caused a significant down-regulation of urate oxidase (10 and 50muM) and a general up-regulation of vitellogenin. A significant reduction of peroxisome relative volumes and smaller peroxisome profiles were observed at 50muM. Co-administration of T and ICI reversed the morphological modifications and vitellogenin levels. The simultaneous exposure of T and F caused a significant and concentration-dependent diminishing in vitellogenin expression. Together, the findings suggest that in the tested model, T acted via both androgen and estrogen receptors to shape the peroxisomal related targets.
Iron chloride catalysed PCDD/F-formation: Experiments and PCDD/F-signatures.[Pubmed:29031055]
Chemosphere. 2018 Jan;191:72-80.
Iron chloride is often cited as catalyst of PCDD/F-formation, together with copper chloride. Conversely, iron chloride catalysis has been less studied during de novo tests. This paper presents such de novo test data, derived from model fly ash incorporating iron (III) chloride and established over a vast range of temperature and oxygen concentration in the gas phase. Both PCDD/F-output and its signature are extensively characterised, including homologue and congener profiles. For the first time, a complete isomer-specific analysis is systematically established, for all samples. Special attention is paid to the chlorophenols route PCDD/F, to the 2,3,7,8-substituted congeners, and to their relationship and antagonism.
Viscum articulatum Burm. f.: a review on its phytochemistry, pharmacology and traditional uses.[Pubmed:29034952]
J Pharm Pharmacol. 2018 Feb;70(2):159-177.
OBJECTIVES: The aim of this study was to review and highlight traditional and ethnobotanical uses, phytochemical constituents, IP status, biological activity and pharmacological activity of Viscum articulatum. METHODS: Thorough literature searches were performed on Viscum articulatum, and data were analysed for reported traditional uses, pharmacological activity, phytochemicals present and patents filed. Scientific and patent databases such as PubMed, Science Direct, Google Scholar, Google patents, USPTO and Espacenet were searched using different keywords. KEY FINDINGS: Viscum articulatum has been traditionally used in different parts of the world for treatment of various ailments. Almost all the parts such as leaves, root, stem and bark are having medicinal values and are reported for their uses in Ayurvedic and Chinese system of medicine for the management of various diseases. Modern scientific studies demonstrate efficacy of this plant against hypertension, ulcer, epilepsy, inflammation, wound, nephrotoxicity, HIV, cancer, etc. Major bioactive phytochemicals include oleanolic acid, betulinic acid, eriodictyol, naringenin, beta-amyrin acetate, visartisides, etc. CONCLUSIONS: Side effects of allopathic medicines have created a global opportunity, acceptance and demand for phytomedicines. Viscum articulatum could be an excellent source of effective and safe phytomedicine for various ailments if focused translational efforts are undertaken by integrating the existing outcomes of researches.
Cytotoxic Dibohemamines D-F from a Streptomyces Species.[Pubmed:29035560]
J Nat Prod. 2017 Oct 27;80(10):2825-2829.
Three dimeric analogues of bohemamines, dibohemamines D-F (1-3), together with dibohemamine A (4), were isolated from Streptomyces sp. CPCC 200497. Their structures were solved using a combination of mass spectrometry, 1D and 2D NMR spectroscopy, and CD. Dibohemamines D and E were new dimeric analogues of bohemamines, and dibohemamine F was a known compound obtained previously by semisynthesis. Dibohemamine F displayed potent cytotoxicity against cancer cell lines A549 and HepG2 with IC50 values of 1.1 and 0.3 muM, respectively. Dibohemamines D and E showed moderate cytotoxicity against cancer cell lines A549 and HepG2.


