Obscuraminol CCAS# 350484-95-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 350484-95-6 | SDF | Download SDF |
| PubChem ID | 101101126 | Appearance | Oil |
| Formula | C16H31NO | M.Wt | 253.43 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
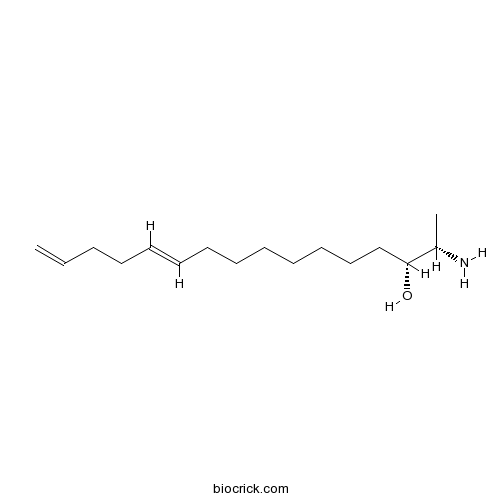
| Chemical Name | (2S,3R,11E)-2-aminohexadeca-11,15-dien-3-ol | ||
| SMILES | CC(C(CCCCCCCC=CCCC=C)O)N | ||
| Standard InChIKey | PKCDBAJESFUNQJ-BUWFCSEKSA-N | ||
| Standard InChI | InChI=1S/C16H31NO/c1-3-4-5-6-7-8-9-10-11-12-13-14-16(18)15(2)17/h3,6-7,15-16,18H,1,4-5,8-14,17H2,2H3/b7-6+/t15-,16+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Tetrahedron, 2001, 57(21):4579-4588.Obscuraminols, new unsaturated amino alcohols from the tunicate Pseudodistoma obscurum: structure and absolute configuration[Reference: WebLink]
|

Obscuraminol C Dilution Calculator

Obscuraminol C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9459 mL | 19.7293 mL | 39.4586 mL | 78.9173 mL | 98.6466 mL |
| 5 mM | 0.7892 mL | 3.9459 mL | 7.8917 mL | 15.7835 mL | 19.7293 mL |
| 10 mM | 0.3946 mL | 1.9729 mL | 3.9459 mL | 7.8917 mL | 9.8647 mL |
| 50 mM | 0.0789 mL | 0.3946 mL | 0.7892 mL | 1.5783 mL | 1.9729 mL |
| 100 mM | 0.0395 mL | 0.1973 mL | 0.3946 mL | 0.7892 mL | 0.9865 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2,5-Dihydroxyxanthone
Catalog No.:BCN7573
CAS No.:35040-32-5
- ent-17-Hydroxykaur-15-en-19-oic acid
Catalog No.:BCN4644
CAS No.:35030-38-7
- Brosimacutin G
Catalog No.:BCN3202
CAS No.:350221-50-0
- NHS-Biotin
Catalog No.:BCC3577
CAS No.:35013-72-0
- Cucurbitadienol
Catalog No.:BCN3342
CAS No.:35012-08-9
- 10beta-Hydroxycadina-4,11(13)-dien-12,8beta-olide
Catalog No.:BCN7435
CAS No.:35001-23-1
- 4alpha,6alpha-Dihydroxyeudesm-11(13)-en-12,8beta-olide
Catalog No.:BCN7431
CAS No.:35001-19-5
- Kurarinone
Catalog No.:BCN2985
CAS No.:34981-26-5
- Kuraridine
Catalog No.:BCN2988
CAS No.:34981-25-4
- Kushenol F
Catalog No.:BCN3798
CAS No.:34981-24-3
- Z-Val-OSu
Catalog No.:BCC2733
CAS No.:3496-11-5
- 3'',4''-Di-O-acetyl-2'',6''-di-O-p-coumaroylastragalin
Catalog No.:BCN6843
CAS No.:349545-02-4
- Obscuraminol D
Catalog No.:BCN1770
CAS No.:350484-99-0
- Obscuraminol E
Catalog No.:BCN1769
CAS No.:350485-00-6
- Obscuraminol F
Catalog No.:BCN1768
CAS No.:350485-01-7
- Obscuraminol B
Catalog No.:BCN1766
CAS No.:350485-82-4
- 4-Epi-curcumenol
Catalog No.:BCN3523
CAS No.:350602-21-0
- Isodemethylwedelolacton
Catalog No.:BCN2766
CAS No.:350681-33-3
- Dendocarbin A
Catalog No.:BCN5287
CAS No.:350986-74-2
- H-D-His-OH
Catalog No.:BCC2959
CAS No.:351-50-8
- Adynerin
Catalog No.:BCN4643
CAS No.:35109-93-4
- GNTI dihydrochloride
Catalog No.:BCC7003
CAS No.:351183-88-5
- Blasticidin S HCl
Catalog No.:BCC5565
CAS No.:3513-03-9
- Deacylmetaplexigenin
Catalog No.:BCC8163
CAS No.:3513-04-0
Serum C-Reactive Protein as a Prognostic Biomarker in Amyotrophic Lateral Sclerosis.[Pubmed:28384752]
JAMA Neurol. 2017 Jun 1;74(6):660-667.
Importance: Various factors have been proposed as possible candidates associated with the prognosis of amyotrophic lateral sclerosis (ALS); however, there is still no consensus on which biomarkers are reliable prognostic factors. C-reactive protein (CRP) is a biomarker of the inflammatory response that shows significant prognostic value for several diseases. Objective: To examine the prognostic significance of CRP in ALS. Design, Setting, and Participants: Patients' serum CRP levels were evaluated from January 1, 2009, to June 30, 2015, in a large cohort of patients with ALS observed by an Italian tertiary multidisciplinary center. Results were replicated in an independent cohort obtained from a population-based registry of patients with ALS. A post hoc analysis was performed of the phase 2 trial of NP001 to determine whether stratification by levels of CRP improves differentiation of responders and nonresponders to the drug. Main Outcomes and Measures: Serum CRP levels from the first examination were recorded to assess their effect on disease progression and survival. Results: A total of 394 patients with ALS (168 women and 226 men; mean [SD] age at diagnosis, 60.18 [13.60] years) were observed in a tertiary multidisciplinary center, and the analysis was replicated in an independent cohort of 116 patients with ALS (50 women and 66 men; mean [SD] age at diagnosis, 67.00 [10.74] years) identified through a regional population-based registry. Serum CRP levels in the 394 patients with ALS correlated with severity of functional impairment, as measured by total score on the ALS Functional Rating Scale-Revised, at first evaluation (r = -0.14818; P = .004), and with patient survival (hazard ratio, 1.129; 95% CI, 1.033-1.234; P = .007). Similar results were found in the independent cohort (hazard ratio, 1.044; 95% CI, 1.016-1.056; P
Discovery of novel 7-azaindole derivatives bearing dihydropyridazine moiety as c-Met kinase inhibitors.[Pubmed:28384549]
Eur J Med Chem. 2017 Jun 16;133:97-106.
A series of 7-azaindole derivatives bearing the dihydropyridazine scaffold were synthesized and evaluated for their c-Met kinase inhibitory, and antiproliferative activity against 4 cancer cell lines (HT29, A549, H460, U87MG) were evaluated in vitro. Most compounds showed moderate to excellent potency. Compared to foretinib, the most promising analog 34 (c-Met IC50: 1.06 nM, a multitarget tyrosine kinase inhibitor) showed a 6.4-, 7.8-, and 3.2-fold increase in activity against HT29, A549, and H460 cell lines, respectively. Structure activity relationship studies indicated that mono-EWGs (such as R2 = F) at 4-position of moiety D was a key factor in improving the antitumor activity.
Effect of water table variations and input of natural organic matter on the cycles of C and N, and mobility of As, Zn and Cu from a soil impacted by the burning of chemical warfare agents: A mesocosm study.[Pubmed:28384583]
Sci Total Environ. 2017 Oct 1;595:279-293.
A mesocosm study was conducted to assess the impact of water saturation episodes and of the input of bioavailable organic matter on the biogeochemical cycles of C and N, and on the behavior of metal(loid)s in a soil highly contaminated by the destruction of arsenical shells. An instrumented mesocosm was filled with contaminated soil taken from the "Place-a-Gaz" site. Four cycles of dry and wet periods of about one month were simulated for 276days. After two dry/wet cycles, organic litter sampled on the site was added above the topsoil. The nitrogen cycle was the most impacted by the wet/dry cycles, as evidenced by a denitrification microbial process in the saturated level. The concentrations of the two most mobile pollutants, Zn and As, in the soil water and in the mesocosm leachate were, respectively, in the 0.3-1.6mM and 20-110muM ranges. After 8months of experiment, about 83g.m(-3) of Zn and 3.5g.m(-3) of As were leached from the soil. These important quantities represent <1% of the solid stock of this contaminant. Dry/wet cycles had no major effect on Zn mobility. However, soil saturation induced the immobilization of As by trapping As V but enhanced As III mobility. These phenomena were amplified by the presence of bioavailable organic matter. The study showed that the natural deposition of forest organic litter allowed a part of the soil's biological function to be restored but did not immobilize all the Zn and As, and even contributed to transport of As III to the surrounding environment. The main hazard of this type of site, contaminated by organo-arsenic chemical weapons, is the constitution of a stock of As that may leach into the surrounding environment for several hundred years.


