Obscuraminol DCAS# 350484-99-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 350484-99-0 | SDF | Download SDF |
| PubChem ID | 101101127 | Appearance | Oil |
| Formula | C16H33NO | M.Wt | 255.44 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
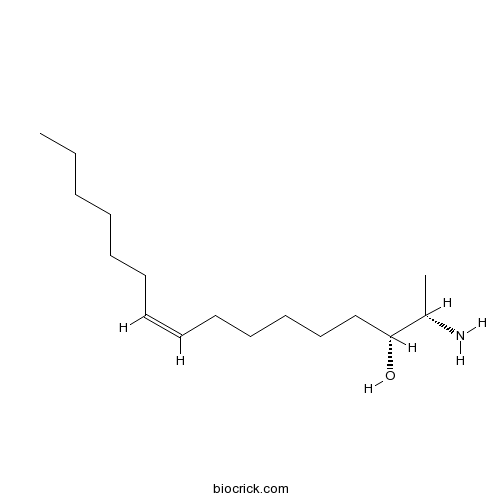
| Chemical Name | (Z,2S,3R)-2-aminohexadec-9-en-3-ol | ||
| SMILES | CCCCCCC=CCCCCCC(C(C)N)O | ||
| Standard InChIKey | SHEYJRIILVVUIW-LWULTOEVSA-N | ||
| Standard InChI | InChI=1S/C16H33NO/c1-3-4-5-6-7-8-9-10-11-12-13-14-16(18)15(2)17/h8-9,15-16,18H,3-7,10-14,17H2,1-2H3/b9-8-/t15-,16+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Tetrahedron, 2001, 57(21):4579-4588.Obscuraminols, new unsaturated amino alcohols from the tunicate Pseudodistoma obscurum: structure and absolute configuration.[Reference: WebLink]
|

Obscuraminol D Dilution Calculator

Obscuraminol D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9148 mL | 19.5741 mL | 39.1481 mL | 78.2963 mL | 97.8703 mL |
| 5 mM | 0.783 mL | 3.9148 mL | 7.8296 mL | 15.6593 mL | 19.5741 mL |
| 10 mM | 0.3915 mL | 1.9574 mL | 3.9148 mL | 7.8296 mL | 9.787 mL |
| 50 mM | 0.0783 mL | 0.3915 mL | 0.783 mL | 1.5659 mL | 1.9574 mL |
| 100 mM | 0.0391 mL | 0.1957 mL | 0.3915 mL | 0.783 mL | 0.9787 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Obscuraminol C
Catalog No.:BCN1767
CAS No.:350484-95-6
- 2,5-Dihydroxyxanthone
Catalog No.:BCN7573
CAS No.:35040-32-5
- ent-17-Hydroxykaur-15-en-19-oic acid
Catalog No.:BCN4644
CAS No.:35030-38-7
- Brosimacutin G
Catalog No.:BCN3202
CAS No.:350221-50-0
- NHS-Biotin
Catalog No.:BCC3577
CAS No.:35013-72-0
- Cucurbitadienol
Catalog No.:BCN3342
CAS No.:35012-08-9
- 10beta-Hydroxycadina-4,11(13)-dien-12,8beta-olide
Catalog No.:BCN7435
CAS No.:35001-23-1
- 4alpha,6alpha-Dihydroxyeudesm-11(13)-en-12,8beta-olide
Catalog No.:BCN7431
CAS No.:35001-19-5
- Kurarinone
Catalog No.:BCN2985
CAS No.:34981-26-5
- Kuraridine
Catalog No.:BCN2988
CAS No.:34981-25-4
- Kushenol F
Catalog No.:BCN3798
CAS No.:34981-24-3
- Z-Val-OSu
Catalog No.:BCC2733
CAS No.:3496-11-5
- Obscuraminol E
Catalog No.:BCN1769
CAS No.:350485-00-6
- Obscuraminol F
Catalog No.:BCN1768
CAS No.:350485-01-7
- Obscuraminol B
Catalog No.:BCN1766
CAS No.:350485-82-4
- 4-Epi-curcumenol
Catalog No.:BCN3523
CAS No.:350602-21-0
- Isodemethylwedelolacton
Catalog No.:BCN2766
CAS No.:350681-33-3
- Dendocarbin A
Catalog No.:BCN5287
CAS No.:350986-74-2
- H-D-His-OH
Catalog No.:BCC2959
CAS No.:351-50-8
- Adynerin
Catalog No.:BCN4643
CAS No.:35109-93-4
- GNTI dihydrochloride
Catalog No.:BCC7003
CAS No.:351183-88-5
- Blasticidin S HCl
Catalog No.:BCC5565
CAS No.:3513-03-9
- Deacylmetaplexigenin
Catalog No.:BCC8163
CAS No.:3513-04-0
- 5-Nonadecylresorcinol
Catalog No.:BCN7629
CAS No.:35176-46-6
Effect of initial GnRH and time of insemination on reproductive performance in cyclic and acyclic beef heifers subjected to a 5-d Co-synch plus progesterone protocol.[Pubmed:29035836]
Theriogenology. 2018 Jan 15;106:39-45.
This study evaluated the effect of initial GnRH and timing of AI in a 5-d Co-synch plus CIDR (device containing 1.38 g of progesterone) protocol on pregnancy per AI (P/AI) and pregnancy loss in beef heifers. A secondary objective was to determine if the effect of initial GnRH on reproductive performance was influenced by cyclicity. Crossbred beef heifers (n = 1068; 301-514 kg of body weight, and 13-15 mo of age) at three locations were assigned to either a 5-d Co-synch plus CIDR protocol with (CIDR5G) or without (CIDR5NG) an initial injection of 100 mug of GnRH at CIDR insertion (Day 0). All heifers received a single dose of 500 mug of cloprostenol at CIDR removal (Day 5) and were divided into two groups to receive GnRH and TAI at either 66 or 72 h (Day 8) after CIDR removal. All heifers were inseminated by one technician with frozen-thawed semen from 1 of 4 sires available commercially. Transrectal ultrasonography was performed on Day 0 to determine cyclicity (presence of CL) and normalcy of the reproductive track, and 27 d after TAI to determine pregnancy status. Non-pregnant heifers (n = 470) were assigned to either a CIDR5G or a CIDR5NG protocol with TAI at 72 h after CIDR removal. Twelve days after second AI, heifers were exposure to bulls for 20 d and pregnancy diagnoses were performed approximately 30 d after second TAI and 60 d after bulls were removed to diagnose bull pregnancies and determine pregnancy loss rate. The percentage of acyclic heifers was 20.3%. Overall P/AI after first TAI was 55.6% (594/1068) and did not differ between CIDR5G and CIDR5NG (56.1 vs. 55.1%), or between TAI66 and TAI72 (55.8 vs. 55.4%). However, cyclic heifers were more likely to become pregnant than acyclic ones (59.3 vs. 41.2%; P < 0.01). Moreover, acyclic heifers subjected to the CIDR5NG had fewer P/AI than those subjected to CIDR5G (P < 0.01). Overall P/AI after resynchronization was 55.1% and did not differ between CIDR5G and CIDR5NG (51.3 vs. 59.0%). Overall pregnancy loss after first and second TAI were 3.0% (18/594) and 3.9% (8/205), respectively. When pregnancy loss data were combined, synchronization protocol (4.1 vs. 2.3% for CIDR5NG and CIDR5G; P = 0.01), cyclicity (5.8 vs. 2.9% for acyclic and cyclic; P = 0.03) and the interaction between synchronization protocol and cyclicity (P = 0.04) were significant. The overall cumulative pregnancy at the end of the breeding season was 94.2% (1006/1068); acyclic heifers were less likely to be pregnant at the end of the breeding season (88.4 vs. 95.8%; P < 0.01). In summary, the initial GnRH administration in a 5-d Co-synch plus CIDR protocol that includes a single PGF treatment is necessary in acyclic beef heifers to optimize P/AI, but not in cyclic heifers. Moreover, omission of initial GnRH was associated to greater pregnancy losses, particularly in acyclic heifers. Timing of AI did not affect P/AI.
Analysis of transient hypermorphic activity of E(spl)D during R8 specification.[Pubmed:29036187]
PLoS One. 2017 Oct 16;12(10):e0186439.
Drosophila atonal (ato) is required for the specification of founding R8 photoreceptors during retinal development. ato is regulated via dual eye-specific enhancers; ato-3' is subject to initial induction whereas 5'-ato facilitates Notch-mediated autoregulation. Notch is further utilized to induce bHLH repressors of the E(spl) locus to restrict Ato from its initial broad expression to individual cells. Although Notch operates in two, distinct phases, it has remained unclear how the two phases maintain independence from one another. The difference in these two phases has attributed to the hypothesized delayed expression of E(spl). However, immunofluorescence data indicate that E(spl) are expressed during early Ato patterning, suggesting a more sophisticated underlying mechanism. To probe this mechanism, we provide evidence that although E(spl) exert no influence on ato-3', E(spl) repress 5'-ato and deletion of the E(spl) locus elicits precocious 5'-ato activity. Thus, E(spl) imposes a delay to the timing in which Ato initiates autoregulation. We next sought to understand this finding in the context of E(spl)D, which encodes a dysregulated variant of E(spl)M8 that perturbs R8 patterning, though, as previously reported, only in conjunction with the mutant receptor Nspl. We established a genetic interaction between E(spl)D and roughened eye (roe), a known modulator of Notch signaling in retinogenesis. This link further suggests a dosage-dependence between E(spl) and the proneural activators Ato and Sens, as indicated via interaction assays in which E(spl)D renders aberrant R8 patterning in conjunction with reduced proneural dosage. In total, the biphasicity of Notch signaling relies, to some degree, on the post-translational regulation of individual E(spl) members and, importantly, that post-translational regulation is likely necessary to modulate the level of E(spl) activity throughout the progression of Ato expression.
Progranulin-mediated deficiency of cathepsin D results in FTD and NCL-like phenotypes in neurons derived from FTD patients.[Pubmed:29036611]
Hum Mol Genet. 2017 Dec 15;26(24):4861-4872.
Frontotemporal dementia (FTD) encompasses a group of neurodegenerative disorders characterized by cognitive and behavioral impairments. Heterozygous mutations in progranulin (PGRN) cause familial FTD and result in decreased PGRN expression, while homozygous mutations result in complete loss of PGRN expression and lead to the neurodegenerative lysosomal storage disorder neuronal ceroid lipofuscinosis (NCL). However, how dose-dependent PGRN mutations contribute to these two different diseases is not well understood. Using iPSC-derived human cortical neurons from FTD patients harboring PGRN mutations, we demonstrate that PGRN mutant neurons exhibit decreased nuclear TDP-43 and increased insoluble TDP-43, as well as enlarged electron-dense vesicles, lipofuscin accumulation, fingerprint-like profiles and granular osmiophilic deposits, suggesting that both FTD and NCL-like pathology are present in PGRN patient neurons as compared to isogenic controls. PGRN mutant neurons also show impaired lysosomal proteolysis and decreased activity of the lysosomal enzyme cathepsin D. Furthermore, we find that PGRN interacts with cathepsin D, and that PGRN increases the activity of cathepsin D but not cathepsins B or L. Finally, we show that granulin E, a cleavage product of PGRN, is sufficient to increase cathepsin D activity. This functional relationship between PGRN and cathepsin D provides a possible explanation for overlapping NCL-like pathology observed in patients with mutations in PGRN or CTSD, the gene encoding cathepsin D. Together, our work identifies PGRN as an activator of lysosomal cathepsin D activity, and suggests that decreased cathepsin D activity due to loss of PGRN contributes to both FTD and NCL pathology in a dose-dependent manner.
Association of Maternal Serum 25-Hydroxyvitamin D Concentrations with Risk of Gestational Anemia.[Pubmed:29035877]
Cell Physiol Biochem. 2017;43(4):1526-1532.
BACKGROUND/AIMS: Vitamin D deficiency has been shown to be associated with a greater prevalence of anemia in various healthy and diseased populations by a great deal of observational studies. However, less work has been done to explore this association in pregnant women. The aim of this study was to evaluate the association between maternal serum 25-hydroxyvitamin D [25(OH)D] concentrations and risk of gestational anemia in a large, nested case-control study. METHODS: The serum 25(OH)D concentrations was measured by enzyme immunoassay in 775 pregnant women affected with anemia and 1550 controls. Logistic regression analysis was conducted to assess the association of 25(OH)D concentrations with risk of gestational anemia. RESULTS: We found the 25(OH)D concentrations was significantly lower in women affected with anemia than in controls. Logistic regression analyses showed that women with 25(OH)D concentrations < 25.0 nmol/L, from 25.0 to 37.4 nmol/L and from 37.5 to 49.9 nmol/L all had increased risk of anemia when compared with women with concentrations from 50.0 to 74.9 nmol/L. And the risk of anemia was significantly increased with the decreasing concentrations of the serum 25(OH)D in a dose-dependent manner (P for trend = 0.012). For women with concentrations < 50.0 nmol/L, they had an 80% increase in anemia risk (95% CI = 1.45-2.25) after adjustment for confounders. We also observed a nonlinear relationship between the serum 25(OH)D and anemia, with a threshold for 25(OH)D of 50.0 nmol/L existed for anemia. CONCLUSION: Maternal serum 25(OH)D < 50.0 nmol/L may be a risk factor for gestational anemia, and it should be monitored for the high-risk pregnant women.


