Dendocarbin ACAS# 350986-74-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 350986-74-2 | SDF | Download SDF |
| PubChem ID | 10911949 | Appearance | Powder |
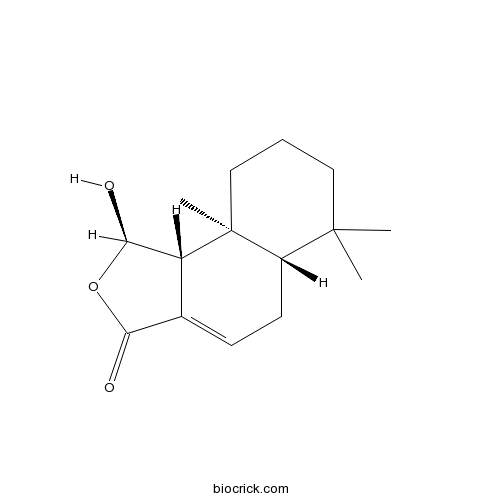
| Formula | C15H22O3 | M.Wt | 250.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,5aS,9aS,9bR)-1-hydroxy-6,6,9a-trimethyl-5,5a,7,8,9,9b-hexahydro-1H-benzo[e][2]benzofuran-3-one | ||
| SMILES | CC1(CCCC2(C1CC=C3C2C(OC3=O)O)C)C | ||
| Standard InChIKey | SNBMCLVOVBJJOU-MDHDOXDCSA-N | ||
| Standard InChI | InChI=1S/C15H22O3/c1-14(2)7-4-8-15(3)10(14)6-5-9-11(15)13(17)18-12(9)16/h5,10-11,13,17H,4,6-8H2,1-3H3/t10-,11+,13+,15-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| In vitro | Ugandenial A, a new drimane-type sesquiterpenoid from Warburgia ugandensis.[Pubmed: 19924033]Molecules. 2009 Sep 28;14(10):3844-50.
|
| Structure Identification | Acta Crystallogr C Struct Chem. 2015 Apr;71(Pt 4):294-7.A monoclinic form of dendocarbin A: a borderline case of one-dimensional isostructural polymorphism.[Pubmed: 25836288]The title compound, Dendocarbin A [systematic name: (1R,5aS,9aS,9bR)-1-hydroxy-6,6,9a-trimethyldodecahydronaphtho[1,2-c]furan-3-one], C15H22O3, is a sesquiterpene lactone isolated from Drimys winteri var chilensis.
Acta Crystallogr C Struct Chem. 2014 Nov;70(Pt 11):1007-10.Dendocarbin A: a sesquiterpene lactone from Drimys winteri.[Pubmed: 25370095]The natural compound Dendocarbin A, C15H22O3, is a sesquiterpene lactone isolated for the first time from Drimys winteri for var chilensis.
|

Dendocarbin A Dilution Calculator

Dendocarbin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9952 mL | 19.976 mL | 39.9521 mL | 79.9041 mL | 99.8801 mL |
| 5 mM | 0.799 mL | 3.9952 mL | 7.9904 mL | 15.9808 mL | 19.976 mL |
| 10 mM | 0.3995 mL | 1.9976 mL | 3.9952 mL | 7.9904 mL | 9.988 mL |
| 50 mM | 0.0799 mL | 0.3995 mL | 0.799 mL | 1.5981 mL | 1.9976 mL |
| 100 mM | 0.04 mL | 0.1998 mL | 0.3995 mL | 0.799 mL | 0.9988 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isodemethylwedelolacton
Catalog No.:BCN2766
CAS No.:350681-33-3
- 4-Epi-curcumenol
Catalog No.:BCN3523
CAS No.:350602-21-0
- Obscuraminol B
Catalog No.:BCN1766
CAS No.:350485-82-4
- Obscuraminol F
Catalog No.:BCN1768
CAS No.:350485-01-7
- Obscuraminol E
Catalog No.:BCN1769
CAS No.:350485-00-6
- Obscuraminol D
Catalog No.:BCN1770
CAS No.:350484-99-0
- Obscuraminol C
Catalog No.:BCN1767
CAS No.:350484-95-6
- 2,5-Dihydroxyxanthone
Catalog No.:BCN7573
CAS No.:35040-32-5
- ent-17-Hydroxykaur-15-en-19-oic acid
Catalog No.:BCN4644
CAS No.:35030-38-7
- Brosimacutin G
Catalog No.:BCN3202
CAS No.:350221-50-0
- NHS-Biotin
Catalog No.:BCC3577
CAS No.:35013-72-0
- Cucurbitadienol
Catalog No.:BCN3342
CAS No.:35012-08-9
- H-D-His-OH
Catalog No.:BCC2959
CAS No.:351-50-8
- Adynerin
Catalog No.:BCN4643
CAS No.:35109-93-4
- GNTI dihydrochloride
Catalog No.:BCC7003
CAS No.:351183-88-5
- Blasticidin S HCl
Catalog No.:BCC5565
CAS No.:3513-03-9
- Deacylmetaplexigenin
Catalog No.:BCC8163
CAS No.:3513-04-0
- 5-Nonadecylresorcinol
Catalog No.:BCN7629
CAS No.:35176-46-6
- Isopropylidenylacetyl-marmesin
Catalog No.:BCN6792
CAS No.:35178-20-2
- INCA-6
Catalog No.:BCC2462
CAS No.:3519-82-2
- D-Tetrahydropalmatine
Catalog No.:BCN2334
CAS No.:3520-14-7
- JC-1
Catalog No.:BCC1669
CAS No.:3520-43-2
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- 4,5-Dimethoxy-1-cyanobenzocyclobutane
Catalog No.:BCC8665
CAS No.:35202-54-1
Ugandenial A, a new drimane-type sesquiterpenoid from Warburgia ugandensis.[Pubmed:19924033]
Molecules. 2009 Sep 28;14(10):3844-50.
One new drimane-type sesquiterpenoid, named ugandenial A (1), was isolated from the ethyl acetate extract of the bark of Warburgia ugandensis (Canellaceae) together with eight known drimane-type sesquiterpenoids: 11alpha-hydroxycinnamosmolide (2), warburganal (3), polygodial (4), mukaadial (5), Dendocarbin A (6), 9alpha-hydroxycinnamolide (7), dendocarbin L (8) and dendocarbin M (9). Their structures were established by detailed spectroscopic analysis. In addition a keto-enol equilibrium was demonstrated for compound 1 through a detailed NMR analysis run in CD(2)Cl(2) at 190 K. Cytotoxicity of the isolated compounds against KB cells was evaluated.
Dendocarbin A: a sesquiterpene lactone from Drimys winteri.[Pubmed:25370095]
Acta Crystallogr C Struct Chem. 2014 Nov;70(Pt 11):1007-10.
The natural compound Dendocarbin A, C15H22O3, is a sesquiterpene lactone isolated for the first time from Drimys winteri for var chilensis. The compound crystallizes in the orthorhombic space group P2(1)2(1)2(1) and its X-ray crystal structure confirmed the S/R character of the chiral centres at C-5/C-10 and C-9/C-11, respectively. The alpha-OH group at C-11 was found to be involved in intermolecular hydrogen bonding, defining chains along the <100> 2(1) screw axis.
A monoclinic form of dendocarbin A: a borderline case of one-dimensional isostructural polymorphism.[Pubmed:25836288]
Acta Crystallogr C Struct Chem. 2015 Apr;71(Pt 4):294-7.
The title compound, Dendocarbin A [systematic name: (1R,5aS,9aS,9bR)-1-hydroxy-6,6,9a-trimethyldodecahydronaphtho[1,2-c]furan-3-one], C15H22O3, is a sesquiterpene lactone isolated from Drimys winteri var chilensis. The monoclinic phase described herein displays an identical molecular structure to the orthorhombic phase that we reported previously [Paz Robles et al. (2014). Acta Cryst. C70, 1007-1010], while varying significantly in chain pitch, and can thus be considered as a borderline case of one-dimensional isostructural polymorphism.


