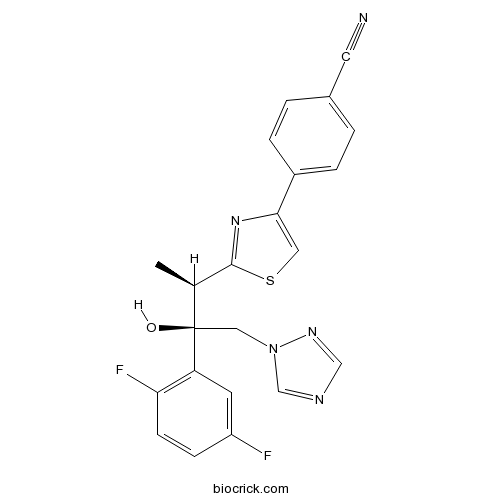
IsavuconazoleCAS# 241479-67-4 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 241479-67-4 | SDF | Download SDF |
| PubChem ID | 6918485 | Appearance | Powder |
| Formula | C22H17F2N5OS | M.Wt | 437.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (114.29 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-[2-[(2R,3R)-3-(2,5-difluorophenyl)-3-hydroxy-4-(1,2,4-triazol-1-yl)butan-2-yl]-1,3-thiazol-4-yl]benzonitrile | ||
| SMILES | CC(C1=NC(=CS1)C2=CC=C(C=C2)C#N)C(CN3C=NC=N3)(C4=C(C=CC(=C4)F)F)O | ||
| Standard InChIKey | DDFOUSQFMYRUQK-RCDICMHDSA-N | ||
| Standard InChI | InChI=1S/C22H17F2N5OS/c1-14(21-28-20(10-31-21)16-4-2-15(9-25)3-5-16)22(30,11-29-13-26-12-27-29)18-8-17(23)6-7-19(18)24/h2-8,10,12-14,30H,11H2,1H3/t14-,22+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isavuconazole Dilution Calculator

Isavuconazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2859 mL | 11.4294 mL | 22.8587 mL | 45.7174 mL | 57.1468 mL |
| 5 mM | 0.4572 mL | 2.2859 mL | 4.5717 mL | 9.1435 mL | 11.4294 mL |
| 10 mM | 0.2286 mL | 1.1429 mL | 2.2859 mL | 4.5717 mL | 5.7147 mL |
| 50 mM | 0.0457 mL | 0.2286 mL | 0.4572 mL | 0.9143 mL | 1.1429 mL |
| 100 mM | 0.0229 mL | 0.1143 mL | 0.2286 mL | 0.4572 mL | 0.5715 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- TMS
Catalog No.:BCC7093
CAS No.:24144-92-1
- Khellactone
Catalog No.:BCN6684
CAS No.:24144-61-4
- 2-CMDO
Catalog No.:BCC5671
CAS No.:24140-98-5
- Flupenthixol dihydrochloride
Catalog No.:BCC7851
CAS No.:2413-38-9
- Adaphostin
Catalog No.:BCC3890
CAS No.:241127-58-2
- Sibiricaxanthone B
Catalog No.:BCN2784
CAS No.:241125-81-5
- Sibiricose A6
Catalog No.:BCN2786
CAS No.:241125-75-7
- 6-Isopentenyloxyisobergapten
Catalog No.:BCC8110
CAS No.:24099-29-4
- Digiferruginol
Catalog No.:BCN3450
CAS No.:24094-45-9
- 5-Chlorothiophene-2-carboxylic acid
Catalog No.:BCC8745
CAS No.:24065-33-6
- Isocurcumenol
Catalog No.:BCN3526
CAS No.:24063-71-6
- Salvicine
Catalog No.:BCN3163
CAS No.:240423-23-8
- Catalpol
Catalog No.:BCN5094
CAS No.:2415-24-9
- N-(4-Methylphenyl)-3-oxobutanamide
Catalog No.:BCC9058
CAS No.:2415-85-2
- Febrifugine
Catalog No.:BCN3269
CAS No.:24159-07-7
- Trametenolic acid
Catalog No.:BCN3330
CAS No.:24160-36-9
- Epitulipinolide
Catalog No.:BCN5095
CAS No.:24164-13-4
- Kobusone
Catalog No.:BCN5096
CAS No.:24173-71-5
- Zoniporide dihydrochloride
Catalog No.:BCC7461
CAS No.:241799-10-0
- H-Lys(Boc)-OH
Catalog No.:BCC2982
CAS No.:2418-95-3
- H-Glu(OtBu)-OH
Catalog No.:BCC2933
CAS No.:2419-56-9
- Boc-Glu-OH
Catalog No.:BCC3386
CAS No.:2419-94-5
- Epiyangambin
Catalog No.:BCN7029
CAS No.:24192-64-1
- Bis(4-hydroxy-3-methylphenyl) sulfide
Catalog No.:BCC8885
CAS No.:24197-34-0
Phase I trial to investigate the effect of renal impairment on isavuconazole pharmacokinetics.[Pubmed:28271239]
Eur J Clin Pharmacol. 2017 Jun;73(6):669-678.
PURPOSE: The purpose of the study is to evaluate the effect of renal impairment (RI) and end-stage renal disease (ESRD) on the pharmacokinetics (PK) of Isavuconazole and the inactive cleavage product, BAL8728. METHODS: A single intravenous dose of the prodrug isavuconazonium sulfate (372 mg, equivalent to 200 mg Isavuconazole and 75 mg of BAL8728 cleavage product) was administered to healthy controls (parts 1 and 2) and participants with mild, moderate, or severe RI (part 2) or ESRD (part 1); ESRD participants received two doses of 200 mg Isavuconazole, 1 h post-dialysis (day 1) and prior to dialysis (day 15). Plasma PK parameters for Isavuconazole included maximum concentration (C max), area under the concentration-time curve (AUC) from time of dose to 72 h (AUC72), AUC extrapolated to infinity (AUCinfinity), AUC to last measurable concentration (AUClast), half-life (t (1/2) h), volume of distribution (V z), and total clearance (CL), for the healthy control group versus those with mild, moderate, or severe RI or ESRD. RESULTS: Isavuconazole C max values were 4% higher in mild RI and 7, 14, and 21% lower in participants with moderate RI, severe RI, or ESRD versus the healthy control group, respectively. When hemodialysis occurred post-dose (day 15), participants with ESRD had a 30% increase in AUC72 for Isavuconazole in parallel with reduction of extracellular volume induced by dialysis. Exposure (AUCinfinity and AUClast) was not significantly different for participants with mild, moderate, or severe RI versus healthy controls although there was considerable variability. The t1/2 (day 1) was 125.5 +/- 63.6 h (healthy control group), 204.5 +/- 82.6 h (ESRD group) in part 1, and 140.5 +/- 77.7 h (healthy control group), 117.0 +/- 66.2 h (mild RI), 158.5 +/- 56.4 h (moderate RI), and 145.8 +/- 65.8 L/h (severe RI) in part 2. CL was 2.4 +/- 0.8 L/h (healthy control group) and 2.9 +/- 1.3 L/h (ESRD group) in part 1 and 2.4 +/- 1.2 L/h (healthy control group), 2.5 +/- 1.0 L/h (mild RI), 2.2 +/- 0.8 L/h (moderate RI), and 2.4 +/- 0.8 L/h (severe RI) in part 2. The V z was 382.6 +/- 150.6 L in the healthy control group and 735.6 +/- 277.3 L in ESRD patients on day 1 in part 1 of the study. In part 2 of the study, V z was 410.8 +/- 89.7 L in the healthy control group, 341.6 +/- 72.3 L in mild RI, 509.1 +/- 262.2 L in moderate RI, and 439.4 L in severe RI. CONCLUSIONS: Based on the findings of this study, dose adjustments of Isavuconazole are unlikely to be required in individuals with RI or in those with ESRD who receive hemodialysis.
Evaluation of the in vitro activity of isavuconazole and comparator voriconazole against 2635 contemporary clinical Candida and Aspergillus isolates.[Pubmed:28373148]
Clin Microbiol Infect. 2017 Nov;23(11):882-887.
OBJECTIVE: The in vitro activity of Isavuconazole was determined for 1677 Candida and 958 Aspergillus isolates from 2012 to 2014 with voriconazole as comparator. METHODS: Aspergillus isolates were screened for resistance using azole-agar. Aspergillus isolates that screened positive and all Candida isolates underwent EUCAST broth microdilution testing. Isolates were categorized as wild-type (wt) or non-wt, adopting EUCAST epidemiological cut-off values (ECOFFs) (where available) or wt upper limits (wtULs; two two-fold dilutions above the MIC50). The CYP51A gene was sequenced for non-wt Aspergillus fumigatus isolates. Itraconazole and posaconazole MICs were determined for selected Aspergillus isolates with Isavuconazole MIC >/=2 mg/L. RESULTS: Isavuconazole MIC50 (range) (mg/L) against Candida species were: Candida albicans: 4), Candida dubliniensis: 4), Saccharomyces cerevisiae (anamorph: Candida robusta): Isavuconazole/voriconazole MICs were found for C. albicans: 0.8/1.0%, C. dubliniensis: 0/1.8%, C. glabrata: 14.9/9.5%, C. krusei: 2.7/1.4%, C. parapsilosis: 1.7/1.8%, C. tropicalis: 14.3/19.1% and S. cerevisiae: 10.0/0%. Isavuconazole MIC50 (range) (mg/L) against Aspergillus species were: A. fumigatus: 1 (16), Aspergillus niger: 2 (1-8), Aspergillus terreus: 1 (0.25-8), Aspergillus flavus: 1 (0.5-2), Aspergillus nidulans: Isavuconazole/voriconazole MICs were found for 13.7/15.2% A. fumigatus, 4.9/0% A. niger and 48.2/22.2% A. terreus. CONCLUSION: Isavuconazole displayed broad in vitro activity, similar to that of voriconazole. Up to 15% of C. glabrata, C. tropicalis and A. fumigatus isolates were non-wt, reflecting increased resistance at a reference centre and technical issues. Significant CYP51A alterations were reliably detected applying the Isavuconazole breakpoint.
Impact of Mucositis on Absorption and Systemic Drug Exposure of Isavuconazole.[Pubmed:28289034]
Antimicrob Agents Chemother. 2017 May 24;61(6). pii: AAC.00101-17.
Isavuconazonium sulfate is the water-soluble prodrug of Isavuconazole. Population analyses have demonstrated relatively predictable pharmacokinetic (PK) behavior in diverse patient populations. We evaluated the impact of mucositis on the oral Isavuconazole exposure using population PK modeling. This study included patients treated in two phase 3 trials of Isavuconazole, SECURE for treatment of invasive aspergillosis (IA) and other filamentous fungi and VITAL for patients with mucormycosis, invasive fungal disease (IFD) caused by other rare fungi, or IA and renal impairment. Mucositis was reported by site investigators and its impact on oral bioavailability was assessed. Use of the oral formulation was at the discretion of the investigator. Patients with plasma samples collected during the use of isavuconazonium sulfate were included in the construction of population PK model. Of 250 patients included, 56 patients had mucositis at therapy onset or as an adverse event during oral Isavuconazole therapy. Levels of oral bioavailability were comparable, at 98.3% and 99.8%, respectively. The average drug exposures (average area under the curve [AUCave]) calculated from either the mean or median parameter estimates were not different between patients with and without mucositis. Mortality and overall clinical responses were similar between patients receiving oral therapy with and without mucositis. We found that Isavuconazole exposures and clinical outcomes in this subset of patients with mucositis who were able to take oral isavuconazonium sulfate were comparable to those in patients without mucositis, despite the difference in oral bioavailability. Therefore, mucositis may not preclude use of the oral formulation of isavuconazonium sulfate.


