2-CMDOD2-like antagonist. Displays some D4 selectivity CAS# 24140-98-5 |
- (24R)-MC 976
Catalog No.:BCC1289
CAS No.:112828-09-8
- (24S)-MC 976
Catalog No.:BCC1291
CAS No.:112849-14-6
- 1alpha, 25-Dihydroxy VD2-D6
Catalog No.:BCC1299
CAS No.:216244-04-1
- 1alpha, 24, 25-Trihydroxy VD2
Catalog No.:BCC1298
CAS No.:457048-34-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 24140-98-5 | SDF | Download SDF |
| PubChem ID | 71296946 | Appearance | Powder |
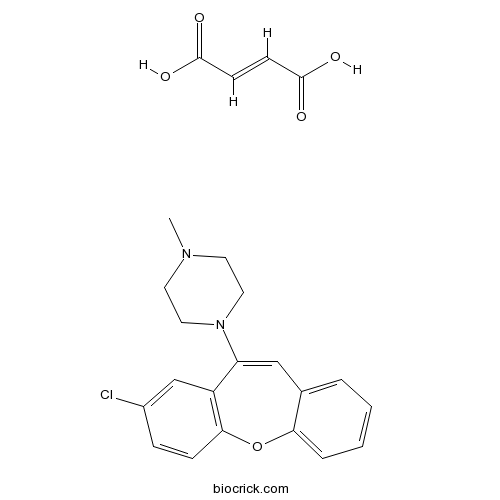
| Formula | C23H23ClN2O5 | M.Wt | 442.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | 2-Chloro-11-(4-methylpiperazino)dib | ||
| SMILES | CN1CCN(CC1)C2=CC3=CC=CC=C3OC4=C2C=C(C=C4)Cl.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | OOHVXDUNWCMZCI-BTJKTKAUSA-N | ||
| Standard InChI | InChI=1S/C19H19ClN2O.C4H4O4/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19;5-3(6)1-2-4(7)8/h2-7,12-13H,8-11H2,1H3;1-2H,(H,5,6)(H,7,8)/b;2-1- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dopamine D2-like receptor antagonist that displays some selectivity for D4 receptors (Ki values are 0.54 and 2.5 nM for D4 and D2 receptors respectively). |

2-CMDO Dilution Calculator

2-CMDO Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2578 mL | 11.2892 mL | 22.5785 mL | 45.1569 mL | 56.4462 mL |
| 5 mM | 0.4516 mL | 2.2578 mL | 4.5157 mL | 9.0314 mL | 11.2892 mL |
| 10 mM | 0.2258 mL | 1.1289 mL | 2.2578 mL | 4.5157 mL | 5.6446 mL |
| 50 mM | 0.0452 mL | 0.2258 mL | 0.4516 mL | 0.9031 mL | 1.1289 mL |
| 100 mM | 0.0226 mL | 0.1129 mL | 0.2258 mL | 0.4516 mL | 0.5645 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Flupenthixol dihydrochloride
Catalog No.:BCC7851
CAS No.:2413-38-9
- Adaphostin
Catalog No.:BCC3890
CAS No.:241127-58-2
- Sibiricaxanthone B
Catalog No.:BCN2784
CAS No.:241125-81-5
- Sibiricose A6
Catalog No.:BCN2786
CAS No.:241125-75-7
- 6-Isopentenyloxyisobergapten
Catalog No.:BCC8110
CAS No.:24099-29-4
- Digiferruginol
Catalog No.:BCN3450
CAS No.:24094-45-9
- 5-Chlorothiophene-2-carboxylic acid
Catalog No.:BCC8745
CAS No.:24065-33-6
- Isocurcumenol
Catalog No.:BCN3526
CAS No.:24063-71-6
- Salvicine
Catalog No.:BCN3163
CAS No.:240423-23-8
- H-Glu-pNA
Catalog No.:BCC2923
CAS No.:24032-35-7
- Agathadiol diacetate
Catalog No.:BCN5093
CAS No.:24022-13-7
- 2-Amino-3-benzyloxypyridine
Catalog No.:BCC8524
CAS No.:24016-03-3
- Khellactone
Catalog No.:BCN6684
CAS No.:24144-61-4
- TMS
Catalog No.:BCC7093
CAS No.:24144-92-1
- Isavuconazole
Catalog No.:BCC5515
CAS No.:241479-67-4
- Catalpol
Catalog No.:BCN5094
CAS No.:2415-24-9
- N-(4-Methylphenyl)-3-oxobutanamide
Catalog No.:BCC9058
CAS No.:2415-85-2
- Febrifugine
Catalog No.:BCN3269
CAS No.:24159-07-7
- Trametenolic acid
Catalog No.:BCN3330
CAS No.:24160-36-9
- Epitulipinolide
Catalog No.:BCN5095
CAS No.:24164-13-4
- Kobusone
Catalog No.:BCN5096
CAS No.:24173-71-5
- Zoniporide dihydrochloride
Catalog No.:BCC7461
CAS No.:241799-10-0
- H-Lys(Boc)-OH
Catalog No.:BCC2982
CAS No.:2418-95-3
- H-Glu(OtBu)-OH
Catalog No.:BCC2933
CAS No.:2419-56-9
L-745,870 suppresses the nighttime serotonin N-acetyltransferase activity in chick retina: in vivo evidence for agonist activity at D4-dopamine receptors.[Pubmed:12658371]
J Neural Transm (Vienna). 2003 Mar;110(3):219-27.
This study examined the in vivo activity of L-745,870 at dopamine (DA) D(4) receptors, using the chick retina as a model system. In dark-adapted retinas of various vertebrates, including hen, DA acting via D(4) receptors suppresses melatonin content and activity of serotonin N-acetyltransferase (AA-NAT, a key regulatory enzyme in melatonin synthesis). Systemic administration to chicks of quinpirole (0.1 mg/kg), a high affinity agonist of D(3)/D(4)-DA receptors, potently decreased the nighttime AA-NAT activity of the retina. The quinpirole-evoked decline in the enzyme activity was attenuated by L-745,870 (0.1-10 nmol/eye). In addition to this action, L-745,870 given to chicks either directly into the eye (0.03-10 nmol/eye) or intraperitoneally (0.5-5 mg/kg) decreased the nighttime AA-NAT activity of the retina in a dose-dependent manner. The suppressive effect of L-745,870 on retinal AA-NAT activity was blocked by 2-chloro-11-(4-methylpiperazino)dibenz[ b, f]oxepin, an antagonist of D(4)-DA receptors, but was not affected by raclopride, an antagonist of D(2)/D(3)-DA receptors. Altogether these results indicate that in chicks L-745,870, the potent putative D(4)-DA receptor antagonist, behaves in vivo as a partial D(4) agonist.
Binding of 5H-dibenzo[a,d]cycloheptene and dibenz[b,f]oxepin analogues of clozapine to dopamine and serotonin receptors.[Pubmed:7861418]
J Med Chem. 1995 Feb 17;38(4):708-14.
Series of 5,11-dicarbo- and 11-carbo-5-oxy-10-(1-alkyl-1,2,3,6-tetrahydro-4 pyridinyl) analogues and a 11-carbo-5-oxy-10-(1-methyl-4-piperidinyl) analogue of the atypical antipsychotic agent clozapine were prepared and tested for binding to the dopamine D-2L and D-4 and serotonin S-2A and S-2C receptors. Some of these analogues were found to have dopamine D-2L and D-4 and serotonin S-2A and S-2C receptor binding activities as high as or higher than those of clozapine, indicating that neither the diazepine structure nor the piperazine ring present in clozapine is essential for high antidopamine activity and or for high dopamine D-4 selectivity (Ki for the dopamine D-2L receptor/Ki for the dopamine D-4 receptor). Increasing in the effective size of the alkyl substituent at the tertiary amine nitrogen atom in the 1,2,3,6-tetrahydro-4-pyridinyl moiety in the 5H-dibenzo[a,d]cycloheptene series reduces the affinity for the dopamine D-4 receptor, but in the dibenz[b,f]oxepin series, no significant change in binding affinity to the dopamine D-4 receptor was observed. Equal or slightly higher affinity for the serotonin S-2A and S-2C receptors was observed for the 10-(1-ethyl-1,2,3,6-tetrahydro-4- pyridinyl) analogues in both series, but for the 10-[1,2,3,6-tetrahydro-1-(2-propenyl)-4- pyridinyl] analogues, any favourable steric factor is overshadowed by an unfavorable electronic effect as a result of change in the basicity of the tertiary amino group in the pyridinyl moiety. Replacement of three of the four nitrogen atoms in clozapine with three carbon or two carbon atoms and an oxygen atom and removal of the chlorine atoms gives 10-(1,2,3,6-tetrahydro-1- methyl-4-pyridinyl)dibenzo[a,d]cycloheptene and 10-(1-methyl-4-piperidinyl)dibenz[b,f]oxepin, each having twice the binding activity to the dopamine D-4 receptor as does clozapine and a dopamine D-4 selectivity equal to that of clozapine.
Binding of 5H-dibenzo[b,e][1,4]diazepine and chiral 5H-dibenzo[a,d]cycloheptene analogues of clozapine to dopamine and serotonin receptors.[Pubmed:8064797]
J Med Chem. 1994 Aug 19;37(17):2686-96.
5H-Dibenzo[b,e][1,4]diazepine, dibenz[b,f]oxepin, and 5H-dibenzo[a,d]cycloheptene analogues of clozapine [8-chloro-11-(4-methylpiperazino)-5H- dibenzo[b,e][1,4]diazepine] were evaluated for their binding affinity to dopamine D-1, D-2, and D-4 and serotonin S-2A (5-HT2A), S-2C (5-HT2C) and S-3 (5-HT3) receptors. The diazepine analogues display selective binding to the dopamine D-4 and serotonin S-2A receptors similar to that of clozapine, but none has a dopamine D-4 selectivity (Ki for the dopamine D-2A receptor/Ki for the dopamine D-4 receptor) greater than that of clozapine. All of the oxepin analogues also show substantial binding to the dopamine D-4 and serotonin S-2A receptors with 10-(4-methylpiperazino)dibenz[b,f]oxepin having a dopamine D-4 selectivity greater than that of clozapine. Some of the 5H-dibenzo-[a,d]cycloheptene analogues also show strong binding to both the dopamine D-4 and serotonin S-2A receptors, 5-methyl-10-(4-methylpiperazino)-5H-dibenzo[a,d]cycloheptene having a dopamine D-4 selectivity of 7.8 as compared to 10 for clozapine but a serotonin S-2A selectivity (Ki for the dopamine D-2 receptor/Ki for the serotonin S-2A receptor) of 2.0 as compared to 28 for clozapine. The serotonin S-2A selectivity of 2-chloro-10-(4-methylpiperazino)-5H-dibenzo[a,d]-cycloheptene++ + is 200. As an extension of these studies, chiral 5-substitute 10-(1,2,3,6-tetrahydro-1-methyl-4-pyridinyl)-5H-dibenzo[a,d]cyclohept ene analogues show a substantial enantiospecificity toward dopamine and serotonin receptor subtypes, (R)-(-)-5-methyl compound having a 2-fold higher dopamine D-4 selectivity than its (S)-(+) enantiomer as the result of enhanced binding to the dopamine D-4 receptor rather than diminished binding to the dopamine D-2 receptor. (pRa,pSb)-(+)-5-(2-Propylidene)-10-(1,2,3,6-tetrahydro-1-met hyl- 4-pyridinyl)-5H-dibenzo[a,d]cycloheptene is 17 times more active in binding to the dopamine D-4 receptor than is its pSa,pRb enantiomer while being only 1.5 times more active in binding to the dopamine D-2 receptor.


