NeokadsuraninCAS# 115181-68-5 |
- Kadsulignan L
Catalog No.:BCN3627
CAS No.:163660-06-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115181-68-5 | SDF | Download SDF |
| PubChem ID | 338282 | Appearance | Powder |
| Formula | C23H26O7 | M.Wt | 414.45 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
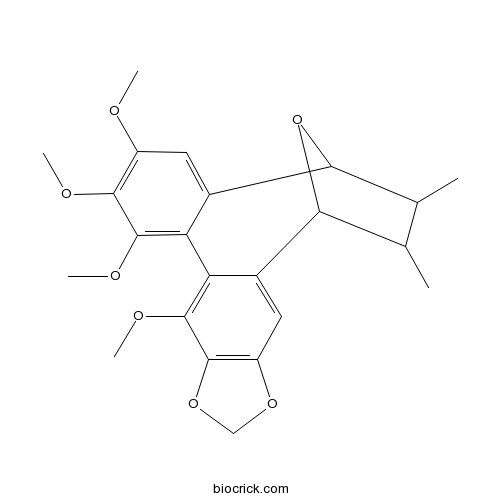
| Chemical Name | 9,12,13,14-tetramethoxy-18,19-dimethyl-5,7,20-trioxapentacyclo[15.2.1.02,10.04,8.011,16]icosa-2,4(8),9,11,13,15-hexaene | ||
| SMILES | CC1C(C2C3=CC(=C(C(=C3C4=C(C5=C(C=C4C1O2)OCO5)OC)OC)OC)OC)C | ||
| Standard InChIKey | RNIZTMIJCBCDBR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H26O7/c1-10-11(2)19-13-8-15-21(29-9-28-15)23(27-6)17(13)16-12(18(10)30-19)7-14(24-3)20(25-4)22(16)26-5/h7-8,10-11,18-19H,9H2,1-6H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Neokadsuranin Dilution Calculator

Neokadsuranin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4128 mL | 12.0642 mL | 24.1284 mL | 48.2567 mL | 60.3209 mL |
| 5 mM | 0.4826 mL | 2.4128 mL | 4.8257 mL | 9.6513 mL | 12.0642 mL |
| 10 mM | 0.2413 mL | 1.2064 mL | 2.4128 mL | 4.8257 mL | 6.0321 mL |
| 50 mM | 0.0483 mL | 0.2413 mL | 0.4826 mL | 0.9651 mL | 1.2064 mL |
| 100 mM | 0.0241 mL | 0.1206 mL | 0.2413 mL | 0.4826 mL | 0.6032 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- R(+)-Gomisin M1
Catalog No.:BCN4362
CAS No.:82467-50-3
- 3-Oxotirucalla-7,24-dien-21-oic acid
Catalog No.:BCN4568
CAS No.:82464-35-5
- Hispanone
Catalog No.:BCN7404
CAS No.:82462-67-7
- Arteannuin A
Catalog No.:BCN4361
CAS No.:82442-48-6
- Pulchinenoside E3
Catalog No.:BCN8187
CAS No.:824401-07-2
- Agomelatine L(+)-Tartaric acid
Catalog No.:BCC4211
CAS No.:824393-18-2
- Maglifloenone
Catalog No.:BCN4360
CAS No.:82427-77-8
- Gomisin M2
Catalog No.:BCN4359
CAS No.:82425-45-4
- Gomisin L2
Catalog No.:BCN7032
CAS No.:82425-44-3
- Gomisin L1
Catalog No.:BCN7039
CAS No.:82425-43-2
- 20-O-Acetylingenol-3-angelate
Catalog No.:BCN8469
CAS No.:82425-35-2
- Ofloxacin
Catalog No.:BCC2526
CAS No.:82419-36-1
- Oroxylin A 7-O-beta-D-glucuronide methyl ester
Catalog No.:BCN1339
CAS No.:82475-01-2
- Baicalin methyl ester
Catalog No.:BCN3252
CAS No.:82475-03-4
- LP 44
Catalog No.:BCC7399
CAS No.:824958-12-5
- A 784168
Catalog No.:BCC6140
CAS No.:824982-41-4
- Pseudolaric Acid B
Catalog No.:BCN6284
CAS No.:82508-31-4
- Pseudolaric acid A
Catalog No.:BCN3717
CAS No.:82508-32-5
- Methyl pseudolarate A
Catalog No.:BCN4363
CAS No.:82508-33-6
- Methyl pseudolarate B
Catalog No.:BCN4364
CAS No.:82508-34-7
- Demethoxydeacetoxypseudolaric acid B
Catalog No.:BCN4365
CAS No.:82508-36-9
- Deacetylpseudolaric acid A
Catalog No.:BCN4366
CAS No.:82508-37-0
- 10-Hydroxy-16-epiaffinine
Catalog No.:BCN4001
CAS No.:82513-70-0
- 5-Methyl-7-methoxyisoflavone
Catalog No.:BCN8465
CAS No.:82517-12-2
Countercurrent Chromatographic Separation of Lipophilic Ascorbic Acid Derivatives and Extract from Kadsura Coccinea Using Hydrophobic Organic-Aqueous Two-Phase Solvent Systems.[Pubmed:20046934]
J Liq Chromatogr Relat Technol. 2009 Jan 1;32(16):2361-2371.
Countercurrent chromatographic (CCC) separation of lipophilic ascorbic acid derivatives and the crude extract from Kadsura Coccinea was performed using the type-J multilayer coil planet centrifuge with a hydrophobic organic-aqueous two-phase solvent system composed of n-hexane/ethyl acetate/ethanol/aqueous 0.1% trifluoroacetic acid at the volume ratio of (5 : 5 : 6 : 2). The lipophilic ascorbic acid derivatives were separated in the order of L-ascrobyl 2,6-dibutyrate, L-ascorbyl 6-palmitate and L-ascorbyl 6-stearate by eluting the lower phase as the mobile phase, and L-ascorbyl 2,6-dipalmitate was separated by eluting the upper phase at the opposite direction. The above solvent system was then applied to the CCC separation of the extract prepared from K. coccinea. With lower phase mobile, the extract was mainly separated into two peaks corresponding to lignans and triterpenoids accordingly. The HPLC analysis of the fractions showed that the former peak contained Kadsulignan N, Schizandrin H and Neokadsuranin as lignans, and the latter peak, Micranoic acid A, Neokadsuranic acid B and beta-Sitosterol as triterpenoids. The overall results indicate that the hydrophobic organic-aqueous two-phase solvent system used in the present studies was useful for the CCC separation of lignans and triterpenoids present in the natural products.
Interiotherins C and D, two new lignans from Kadsura interior and antitumor-promoting effects of related neolignans on Epstein-Barr virus activation.[Pubmed:12350139]
J Nat Prod. 2002 Sep;65(9):1242-5.
Two new lignans, interiotherins C (1) and D (2), together with the known compounds interiorin (3), heteroclitin F (4), Neokadsuranin (5), heteroclitin D (6), kadsurin (7), gomisin A (8), schisandrin C (9), interiotherin A (10), angeloylgomisin R (11), gomisin G (12), interiotherin B (13), and gomisin C (14), were isolated from the stems of Kadsura interior. The structures and stereochemistries of the new compounds were determined from mass, CD, and NMR spectral data. Fourteen neolignans were screened as potential antitumor promoters by examining their ability to inhibit Epstein-Barr virus early antigen (EBV-EA) activation (induced by 12-O-tetradecanoylphorbol-13-acetate) in Raji cells. Neokadsuranin (5) and schisandrin C (9) were the most potent compounds. These data suggest that some neolignans might be valuable antitumor promoters or chemopreventors.
Neokadsuranin, a Tetrahydrofuranoid Dibenzocyclooctadiene Lignan from Stems of Kadsura coccinea.[Pubmed:17265201]
Planta Med. 1988 Feb;54(1):45-6.
A tetrahydrofuranoid dibenzocyclooctadiene lignan, which we name Neokadsuranin, was isolated from the stems of Kadsura coccinea, collected in Kuangxi, China. The absolute configuration was elucidated by spectral analysis.


