Isovouacapenol CCAS# 455255-15-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 455255-15-9 | SDF | Download SDF |
| PubChem ID | 3009285 | Appearance | Powder |
| Formula | C27H34O5 | M.Wt | 438.6 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
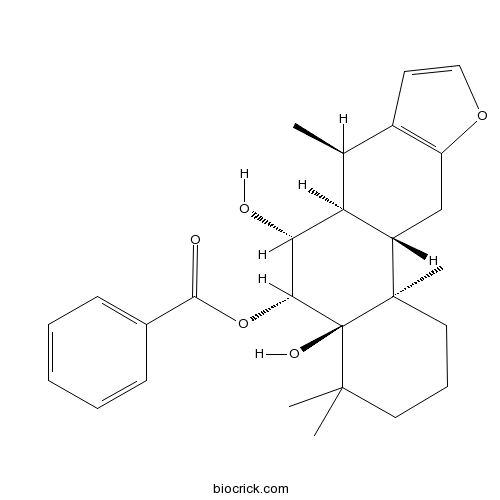
| Chemical Name | [(4aR,5R,6R,6aS,7R,11aS,11bR)-4a,6-dihydroxy-4,4,7,11b-tetramethyl-2,3,5,6,6a,7,11,11a-octahydro-1H-naphtho[2,1-f][1]benzofuran-5-yl] benzoate | ||
| SMILES | CC1C2C(CC3=C1C=CO3)C4(CCCC(C4(C(C2O)OC(=O)C5=CC=CC=C5)O)(C)C)C | ||
| Standard InChIKey | YMUSGWGTHSRGHT-WVTZLOSNSA-N | ||
| Standard InChI | InChI=1S/C27H34O5/c1-16-18-11-14-31-20(18)15-19-21(16)22(28)23(32-24(29)17-9-6-5-7-10-17)27(30)25(2,3)12-8-13-26(19,27)4/h5-7,9-11,14,16,19,21-23,28,30H,8,12-13,15H2,1-4H3/t16-,19-,21-,22+,23+,26+,27+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| Structure Identification | J Nat Prod. 2003 Oct;66(10):1378-81.Cassane diterpenoids of Caesalpinia pulcherrima.[Pubmed: 14575441 ]
Acta Crystallogr Sect E Struct Rep Online. 2010 Jul 17;66(Pt 8):o2059-60.Absolute configuration of isovouacapenol C.[Pubmed: 21588364 ]
|

Isovouacapenol C Dilution Calculator

Isovouacapenol C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.28 mL | 11.3999 mL | 22.7998 mL | 45.5996 mL | 56.9995 mL |
| 5 mM | 0.456 mL | 2.28 mL | 4.56 mL | 9.1199 mL | 11.3999 mL |
| 10 mM | 0.228 mL | 1.14 mL | 2.28 mL | 4.56 mL | 5.7 mL |
| 50 mM | 0.0456 mL | 0.228 mL | 0.456 mL | 0.912 mL | 1.14 mL |
| 100 mM | 0.0228 mL | 0.114 mL | 0.228 mL | 0.456 mL | 0.57 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Benzoyl-3-methyl-1-phenyl-5-pyrazolone
Catalog No.:BCC8695
CAS No.:4551-69-3
- Grossamide K
Catalog No.:BCC4547
CAS No.:
- Corosolic acid
Catalog No.:BCN5503
CAS No.:4547-24-4
- Acetophenone tosylhydrazone
Catalog No.:BCC8804
CAS No.:4545-21-5
- kobe2602
Catalog No.:BCC5291
CAS No.:454453-49-7
- Nudifloside D
Catalog No.:BCN7005
CAS No.:454212-54-5
- PSB 11 hydrochloride
Catalog No.:BCC7239
CAS No.:453591-58-7
- Motesanib
Catalog No.:BCC1776
CAS No.:453562-69-1
- Saprirearine
Catalog No.:BCN3980
CAS No.:453518-30-4
- Boc-Gly-OH
Catalog No.:BCC3396
CAS No.:4530-20-5
- Boc-DL-Phe-OH
Catalog No.:BCC3434
CAS No.:4530-18-1
- GW788388
Catalog No.:BCC3666
CAS No.:452342-67-5
- Zaurategrast
Catalog No.:BCC2070
CAS No.:455264-31-0
- H-Glu(OBzl)-OBzl.HCl
Catalog No.:BCC2927
CAS No.:4561-10-8
- Neocnidilide
Catalog No.:BCN8174
CAS No.:4567-33-3
- 3-Benzyl-5-(2-hydroxyethyl)-4-methylthiazolium chloride
Catalog No.:BCC8625
CAS No.:4568-71-2
- 5-O-Methylgenistein
Catalog No.:BCN7714
CAS No.:4569-98-6
- 1alpha, 24, 25-Trihydroxy VD2
Catalog No.:BCC1298
CAS No.:457048-34-9
- Pyridone 6
Catalog No.:BCC1874
CAS No.:457081-03-7
- PA 452
Catalog No.:BCC8005
CAS No.:457657-34-0
- BMS 470539 dihydrochloride
Catalog No.:BCC7850
CAS No.:457893-92-4
-
Scutebarbatine L
Catalog No.:BCN8369
CAS No.:960302-91-4
- Curcumin
Catalog No.:BCN5504
CAS No.:458-37-7
- Ilicic acid
Catalog No.:BCN5505
CAS No.:4586-68-9
Cassane diterpenoids of Caesalpinia pulcherrima.[Pubmed:14575441]
J Nat Prod. 2003 Oct;66(10):1378-81.
Five new cassane diterpenoids (1-5) were isolated from the roots of Caesalpinia pulcherrima, along with the known Isovouacapenol C (6), pulcherrimin A (11), and 6beta-cinnamoyl-7beta-hydroxyvouacapen-5alpha-ol (12). Compounds 3-5 possess the alpha,beta-butenolide moiety, whereas compounds 1 and 2 contain a more usual 2,3-disubstituted furan unit. Compounds 7 and 8 were derived from hydrolysis of 6, while 9 and 10 were derived from acetylation and oxidation of 6, respectively. The (1)H and (13)C NMR spectra of all compounds were completely assigned using a combination of 2D NMR experiments, including (1)H-(1)H COSY, HSQC, HMBC, and T-ROESY sequences.
Absolute configuration of isovouacapenol C.[Pubmed:21588364]
Acta Crystallogr Sect E Struct Rep Online. 2010 Jul 17;66(Pt 8):o2059-60.
The title compound, C(27)H(34)O(5) {systematic name: (4aR,5R,6R,6aS,7R,11aS,11bR)-4a,6-dihy-droxy-4,4,7,11b-tetra-methyl-1,2,3,4,4a,5, 6,6a,7,11,11a,11b-dodeca-hydro-phenanthro[3,2-b]furan-5-yl benzoate}, is a cassane furan-oditerpene, which was isolated from the roots of Caesalpinia pulcherrima. The three cyclo-hexane rings are trans fused: two of these are in chair conformations with the third in a twisted half-chair conformation, whereas the furan ring is almost planar (r.m.s. deviation = 0.003 A). An intra-molecular C-Hcdots, three dots, centeredO inter-action generates an S(6) ring. The absolute configurations of the stereogenic centres at positions 4a, 5, 6, 6a, 7, 11a and 11b are R, R, R, S, R, S and R, respectively. In the crystal, mol-ecules are linked into infinite chains along [010] by O-Hcdots, three dots, centeredO hydrogen bonds. Ccdots, three dots, centeredO [3.306 (2)-3.347 (2) A] short contacts and C-Hcdots, three dots, centeredpi inter-actions also occur.


