MotesanibInhibitor of Flk-1/Flt-4/PDGFR-/c-Kit CAS# 453562-69-1 |
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Amuvatinib (MP-470, HPK 56)
Catalog No.:BCC2258
CAS No.:850879-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 453562-69-1 | SDF | Download SDF |
| PubChem ID | 11667893 | Appearance | Powder |
| Formula | C22H23N5O | M.Wt | 373.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AMG 706; | ||
| Solubility | DMSO : ≥ 100 mg/mL (267.77 mM) *"≥" means soluble, but saturation unknown. | ||
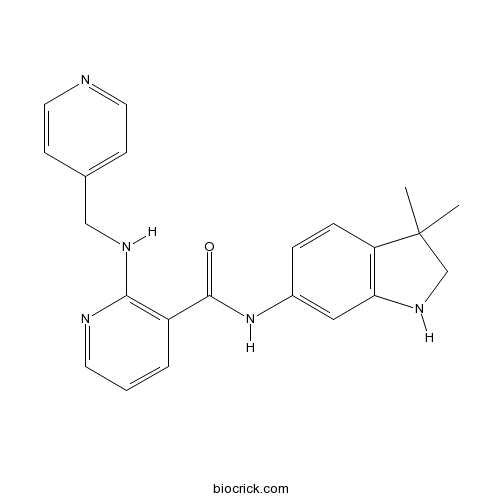
| Chemical Name | N-(3,3-dimethyl-1,2-dihydroindol-6-yl)-2-(pyridin-4-ylmethylamino)pyridine-3-carboxamide | ||
| SMILES | CC1(CNC2=C1C=CC(=C2)NC(=O)C3=C(N=CC=C3)NCC4=CC=NC=C4)C | ||
| Standard InChIKey | RAHBGWKEPAQNFF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H23N5O/c1-22(2)14-26-19-12-16(5-6-18(19)22)27-21(28)17-4-3-9-24-20(17)25-13-15-7-10-23-11-8-15/h3-12,26H,13-14H2,1-2H3,(H,24,25)(H,27,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Motesanib is a potent ATP-competitive inhibitor of VEGFR1/2/3 with IC50 values of 2 nM, 3 nM and 6 nM, respectively. | |||||
| Targets | VEGFR1 | VEGFR2 | VEGFR3 | |||
| IC50 | 2 nM | 3 nM | 6 nM | |||

Motesanib Dilution Calculator

Motesanib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6777 mL | 13.3883 mL | 26.7766 mL | 53.5533 mL | 66.9416 mL |
| 5 mM | 0.5355 mL | 2.6777 mL | 5.3553 mL | 10.7107 mL | 13.3883 mL |
| 10 mM | 0.2678 mL | 1.3388 mL | 2.6777 mL | 5.3553 mL | 6.6942 mL |
| 50 mM | 0.0536 mL | 0.2678 mL | 0.5355 mL | 1.0711 mL | 1.3388 mL |
| 100 mM | 0.0268 mL | 0.1339 mL | 0.2678 mL | 0.5355 mL | 0.6694 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AMG 706 (Motesanib)Description:IC50: Motesanib diphosphate (AMG-706) is a potent ATP-competitive inhibitor of VEGFR1/2/3 with IC50 of 2 nM/3 nM/6 nM, respectively [1].
Vascular endothelial growth factor (VEGF) is an important signaling protein involved in both vasculogenesis (the formation of the circulatory system) and angiogenesis (the growth of blood vessels from pre-existing vasculature). Motesanib (AMG 706) is an orally administered small molecule belonging to angiokinase inhibitor class which acts as an antagonist of VEGF receptors, platelet-derived growth factor receptors, and stem cell factor receptors.
In vitro: Motesanib diphosphate has broad activity against the human VEGFR family, and displays >1000 selectivity against EGFR, Src, and p38 kinase. Motesanib Diphosphate significantly inhibits VEGF-induced cellular proliferation of HUVECs with an IC50 of 10 nM, while displaying little effect at bFGF-induced proliferation with an IC50 of >3,000 nM. Motesanib Diphosphate also potently inhibits PDGF-induced proliferation and SCF-induced c-kit phosphorylation with IC50 of 207 nM and 37 nM, respectively, but not effective against the EGF-induced EGFR phosphorylation and cell viability of A431 cells [1]. Althouth displaying little antiproliferative activity on cell growth of HUVECs alone, Motesanib diphosphate treatment significantly sensitizes the cells to fractionated radiation [2].
In vivo: Oral administration of AMG 706 potently inhibited VEGF-induced angiogenesis in the rat corneal model and induced regression of established A431 xenografts. AMG 706 was well tolerated and had no significant effects o AMG 706n body weight or on the general health of the animals. Histologic analysis of tumor xenografts from AMG 706–treated animals revealed an increase in endothelial apoptosis and a reduction in blood vessel area that preceded an increase in tumor cell apoptosis. In summary, AMG 706 is an orally bioavailable, well-tolerated multikinase inhibitor that is presently under clinical investigation for the treatment of human malignancies [1].
Clinical trial: Motesanib was originally investigated for effectiveness against advanced nonsquamous non-small-cell lung cancer (NSCLC), with Phase II trials indicating an effectiveness comparable to bevacizumab when they were both used in combination with paclitaxel/carboplatin. However a later and more detailed Phase III trial failed to show any benefit for the treatment of NSCLC. A second Phase III trial was started in 2012, which focused on patients from Asian backgrounds (performed on the bases of subgroup analysis) however this also failed to meet its primary endpoint. The drug has undergone a Phase II evaluation as first-line therapy for breast cancer however this study found no evidence to support further investigation. Phase II testing against persistent or recurrent ovarian, fallopian tube and primary peritoneal carcinomas was also unsuccessful. There have also been 2 separate Phase II clinical trials for thyroid cancer which have both shown promising results (http://en.wikipedia.org/wiki/Motesanib).
Reference:
[1] Polverino A, Coxon A, Starnes C, et al. AMG 706, an oral, multikinase inhibitor that selectively targets vascular endothelial growth factor, platelet-derived growth factor, and kit receptors, potently inhibits angiogenesis and induces regression in tumor xenografts. Cancer Res. 2006;66(17):8715-21.
[2] Kruser TJ1 Wheeler DL, Armstrong EA, Iida M, Kozak KR, van der Kogel AJ, Bussink J, Coxon A, Polverino A, Harari PM. Augmentation of radiation response by motesanib, a multikinase inhibitor that targets vascular endothelial growth factor receptors. Clin Cancer Res. 2010;16(14):3639-47.
- Saprirearine
Catalog No.:BCN3980
CAS No.:453518-30-4
- Boc-Gly-OH
Catalog No.:BCC3396
CAS No.:4530-20-5
- Boc-DL-Phe-OH
Catalog No.:BCC3434
CAS No.:4530-18-1
- GW788388
Catalog No.:BCC3666
CAS No.:452342-67-5
- SU14813 double bond Z
Catalog No.:BCC1972
CAS No.:452105-23-6
- Boc-N-Me-Val-OH
Catalog No.:BCC3357
CAS No.:45170-31-8
- AV-412
Catalog No.:BCC5119
CAS No.:451493-31-5
- AV-412 free base
Catalog No.:BCC5120
CAS No.:451492-95-8
- 10-Hydroxydihydroperaksine
Catalog No.:BCN5502
CAS No.:451478-47-0
- H-1152
Catalog No.:BCC1615
CAS No.:451462-58-1
- H-D-Glu(OtBu)-OH
Catalog No.:BCC2942
CAS No.:45125-00-6
- H-Glu-OtBu
Catalog No.:BCC2925
CAS No.:45120-30-7
- PSB 11 hydrochloride
Catalog No.:BCC7239
CAS No.:453591-58-7
- Nudifloside D
Catalog No.:BCN7005
CAS No.:454212-54-5
- kobe2602
Catalog No.:BCC5291
CAS No.:454453-49-7
- Acetophenone tosylhydrazone
Catalog No.:BCC8804
CAS No.:4545-21-5
- Corosolic acid
Catalog No.:BCN5503
CAS No.:4547-24-4
- Grossamide K
Catalog No.:BCC4547
CAS No.:
- 4-Benzoyl-3-methyl-1-phenyl-5-pyrazolone
Catalog No.:BCC8695
CAS No.:4551-69-3
- Isovouacapenol C
Catalog No.:BCN6557
CAS No.:455255-15-9
- Zaurategrast
Catalog No.:BCC2070
CAS No.:455264-31-0
- H-Glu(OBzl)-OBzl.HCl
Catalog No.:BCC2927
CAS No.:4561-10-8
- Neocnidilide
Catalog No.:BCN8174
CAS No.:4567-33-3
- 3-Benzyl-5-(2-hydroxyethyl)-4-methylthiazolium chloride
Catalog No.:BCC8625
CAS No.:4568-71-2
A case report of motesanib-induced biliary sludge formation causing obstructive cholangitis with acute pancreatitis treated by endoscopic sphincterotomy.[Pubmed:27631212]
Medicine (Baltimore). 2016 Sep;95(37):e4645.
BACKGROUND: Gallbladder toxicity was reported in most Motesanib studies with varying frequency and at variable times after initiation of treatment. METHOD AND RESULTS: A 44-year-old man was admitted due to severe epigastric pain. The patient was diagnosed with non-small cell lung cancer 9 months ago and received 6 cycles of chemotherapy with Motesanib, paclitaxel, and carboplatin. Ultrasonography showed a large amount of sludge within gallbladder. Computed tomography scan demonstrated diffuse dilatation of biliary tree with distended gallbladder without evidence of stone and mild pancreatic swelling. Endoscopic retrograde cholangiopancreatography showed yellowish viscous mucoid plug impacting ampullary orifice and dilated bile duct with amorphous filling defect at distal half of common duct. Endoscopic sphincterotomy was performed to prevent biliary obstruction and recurrent pancreatitis after removal of mucoid material. CONCLUSION: To the best of our knowledge, this is the first report of obstructive cholangitis and acute pancreatitis associated with sludge formation during Motesanib therapy. Endoscopic sphincterotomy appears to be useful to treat and prevent biliary obstruction caused by Motesanib-induced biliary sludge.
Correction: challenges in developing a validated biomarker for angiogenesis inhibitors: the motesanib experience.[Pubmed:25811784]
PLoS One. 2015 Mar 26;10(3):e0121162.
[This corrects the article DOI: 10.1371/journal.pone.0108048.].
Population pharmacokinetic modeling of motesanib and its active metabolite, M4, in cancer patients.[Pubmed:27137719]
Clin Pharmacol Drug Dev. 2015 Nov;4(6):463-72.
Motesanib is a small molecule and potent multikinase inhibitor with antiangiogenic and antitumor activity. Population pharmacokinetic (POPPK) modeling of Motesanib and M4, an active metabolite, was performed to assess sources of variability in cancer patients. The analysis included data collected from 451 patients from 8 clinical trials with oral doses of Motesanib ranging from 25 to 175 mg, either once daily or twice daily. The POPPK analyses were performed using nonlinear mixed-effect models with a sequential approach. Covariate effects of demographics and other baseline characteristics were assessed with stepwise covariate modeling. A 2-compartment model with food effect on absorption parameters fitted the PK data of Motesanib well. The effects albumin and sex on apparent clearance (CL/F) of Motesanib were statistically significant. The albumin effect was more important but remained below a 25% difference. A 1-compartment model fitted PK data of M4 well. Effects of race (Asian vs non-Asian) and dosing frequency were identified as statistically significant covariates on the CL/F of M4. The maximum effect of albumin would result in less than 25% change in Motesanib CL/F and as such would not warrant any dosing adjustment. However, faster elimination of M4 in Asian patients requires further investigation.
Effects of a Multikinase Inhibitor Motesanib (AMG 706) Alone and Combined with the Selective DuP-697 COX-2 Inhibitor on Colorectal Cancer Cells.[Pubmed:27039732]
Asian Pac J Cancer Prev. 2016;17(3):1103-10.
In the present study, we investigated the effects of Motesanib (AMG 706), a multikinase inhibitor alone and in combination with DuP-697, an irreversible selective inhibitor of COX-2, on cell proliferation, angiogenesis, and apoptosis induction in a human colorectal cancer cell line (HT29). Real time cell analysis (RTCA, Xcelligence system) was used to determine the effects on colorectal cancer cell proliferation. Apoptosis was assessed with annexin V staining and angiogenesis was determined with chorioallantoic membrane model. We found that Motesanib alone exerted antiproliferative, antiangiogenic and apoptotic effects on HT29 colorectal cancer cells. Combination with DUP-697 increased the antiproliferative, antiangiogenic and apoptotic effects. Results of this study indicate that Motesanib may be a good choice in treatment of colorectal tumors. In addition, the increased effects of combination of Motesanib with DuP-697 raise the possibility of using lower doses of these drugs and therefore avoid/minimize the dose-dependent side effects generally observed.


