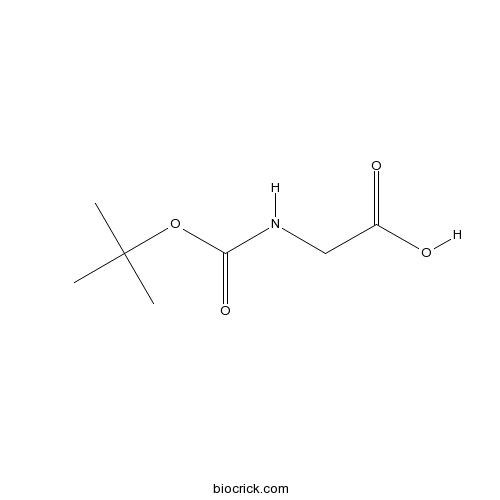
Boc-Gly-OHCAS# 4530-20-5 |
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4530-20-5 | SDF | Download SDF |
| PubChem ID | 78288 | Appearance | Powder |
| Formula | C7H13NO4 | M.Wt | 175.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-[(2-methylpropan-2-yl)oxycarbonylamino]acetic acid | ||
| SMILES | CC(C)(C)OC(=O)NCC(=O)O | ||
| Standard InChIKey | VRPJIFMKZZEXLR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H13NO4/c1-7(2,3)12-6(11)8-4-5(9)10/h4H2,1-3H3,(H,8,11)(H,9,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boc-Gly-OH Dilution Calculator

Boc-Gly-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.7078 mL | 28.5388 mL | 57.0776 mL | 114.1553 mL | 142.6941 mL |
| 5 mM | 1.1416 mL | 5.7078 mL | 11.4155 mL | 22.8311 mL | 28.5388 mL |
| 10 mM | 0.5708 mL | 2.8539 mL | 5.7078 mL | 11.4155 mL | 14.2694 mL |
| 50 mM | 0.1142 mL | 0.5708 mL | 1.1416 mL | 2.2831 mL | 2.8539 mL |
| 100 mM | 0.0571 mL | 0.2854 mL | 0.5708 mL | 1.1416 mL | 1.4269 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Boc-Gly-OH
- Boc-DL-Phe-OH
Catalog No.:BCC3434
CAS No.:4530-18-1
- GW788388
Catalog No.:BCC3666
CAS No.:452342-67-5
- SU14813 double bond Z
Catalog No.:BCC1972
CAS No.:452105-23-6
- Boc-N-Me-Val-OH
Catalog No.:BCC3357
CAS No.:45170-31-8
- AV-412
Catalog No.:BCC5119
CAS No.:451493-31-5
- AV-412 free base
Catalog No.:BCC5120
CAS No.:451492-95-8
- 10-Hydroxydihydroperaksine
Catalog No.:BCN5502
CAS No.:451478-47-0
- H-1152
Catalog No.:BCC1615
CAS No.:451462-58-1
- H-D-Glu(OtBu)-OH
Catalog No.:BCC2942
CAS No.:45125-00-6
- H-Glu-OtBu
Catalog No.:BCC2925
CAS No.:45120-30-7
- H-Aib-OtBu.HCl
Catalog No.:BCC3208
CAS No.:4512-32-7
- H-Met-NH2
Catalog No.:BCC2994
CAS No.:4510-08-1
- Saprirearine
Catalog No.:BCN3980
CAS No.:453518-30-4
- Motesanib
Catalog No.:BCC1776
CAS No.:453562-69-1
- PSB 11 hydrochloride
Catalog No.:BCC7239
CAS No.:453591-58-7
- Nudifloside D
Catalog No.:BCN7005
CAS No.:454212-54-5
- kobe2602
Catalog No.:BCC5291
CAS No.:454453-49-7
- Acetophenone tosylhydrazone
Catalog No.:BCC8804
CAS No.:4545-21-5
- Corosolic acid
Catalog No.:BCN5503
CAS No.:4547-24-4
- Grossamide K
Catalog No.:BCC4547
CAS No.:
- 4-Benzoyl-3-methyl-1-phenyl-5-pyrazolone
Catalog No.:BCC8695
CAS No.:4551-69-3
- Isovouacapenol C
Catalog No.:BCN6557
CAS No.:455255-15-9
- Zaurategrast
Catalog No.:BCC2070
CAS No.:455264-31-0
- H-Glu(OBzl)-OBzl.HCl
Catalog No.:BCC2927
CAS No.:4561-10-8
Synthesis and characterization of new galanthamine derivatives comprising peptide moiety.[Pubmed:19508224]
Protein Pept Lett. 2009;16(9):1024-8. Epub 2009 Sep 1.
New analogues of galanthamine containing peptide fragments either at 6 or 11 position, were synthesized by reaction between galanthamine molecule and dipeptides and tripeptide, derivatives of N-(3,4-dichlorophenyl)-D,L-Ala-OH. The best results according to yields, easily purification of the target products, and simplicity of the scheme realisation was achieved by using of cyanomethyl ester of Boc-Gly-OH as activated compound.
Bovine prothrombin fragment 1, segment 1-10. Synthesis and immunological investigation.[Pubmed:7216619]
Int J Pept Protein Res. 1980 Nov;16(5):440-9.
The N-terminal decapeptide methyl ester, H-Ala-Asn-Lys-Gly-Phe-Leu-Gla-Gla-Val-Arg-OCH3 (16) of bovine prothrombin fragment 1 has been prepared by standard solution techniques, via a fragment coupling strategy. Hexapeptide Boc-Ala-Asn-Lys epsilon (Boc)-Gly-Phe-Leu-OBzl (9) was obtained by coupling Boc-Ala-Asn-Lys epsilon (Boc)Gly-OH (6) to the trifluoroacetate salt of H-Phe-Leu-OBzl (8). Hydrogenolysis of (9) followed by coupling to HCl. H-Gla gamma (OtBu)2-Gla gamma (OtBu)2-Val-Arg(HCl)-OCH3 (14) gave the fully protected decapeptide (15). Treatment of 15 with 90% trifluoroacetic acid followed by ion exchange chromatography of 15 yielded the methyl ester (16). The decapeptide 16 labeled with 125I using the Bolton-Hunter reagent, did not bind to antibodies specific for the calcium ion-induced conformation of bovine fragment 1.
N-urethane-protected amino alkyl isothiocyanates: synthesis, isolation, characterization, and application to the synthesis of thioureidopeptides.[Pubmed:19537728]
J Org Chem. 2009 Aug 7;74(15):5260-6.
Synthetically useful N-Fmoc amino-alkyl isothiocyanates have been described, starting from protected amino acids. These compounds have been synthesized in excellent yields by thiocarbonylation of the monoprotected 1,2-diamines with CS2/TEA/p-TsCl, isolated as stable solids, and completely characterized. The procedure has been extended to the synthesis of amino alkyl isothiocyanates from Boc- and Z-protected amino acids as well. The utility of these isothiocyanates for peptidomimetics synthesis has been demonstrated by employing them in the preparation of a series of dithioureidopeptide esters. Boc-Gly-OH and Boc-Phe-OH derived isothiocyanates 9a and 9c have been obtained as single crystals and their structures solved through X-ray diffraction. They belong to the orthorhombic crystal system, and have a single molecule in the asymmetric unit (Z' = 1). 9a crystallizes in the centrosymmetric space group Pbca, while 9c crystallizes in the noncentrosymmetric space group P2(1)2(1)2(1).


