K-Ras G12C-IN-1CAS# 1629265-17-3 |
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- GF 109203X
Catalog No.:BCC3704
CAS No.:133052-90-1
- Go 6983
Catalog No.:BCC3705
CAS No.:133053-19-7
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1629265-17-3 | SDF | Download SDF |
| PubChem ID | 90462972 | Appearance | Powder |
| Formula | C22H23Cl2N3O3 | M.Wt | 448.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 40 mg/mL (89.22 mM) *"≥" means soluble, but saturation unknown. | ||
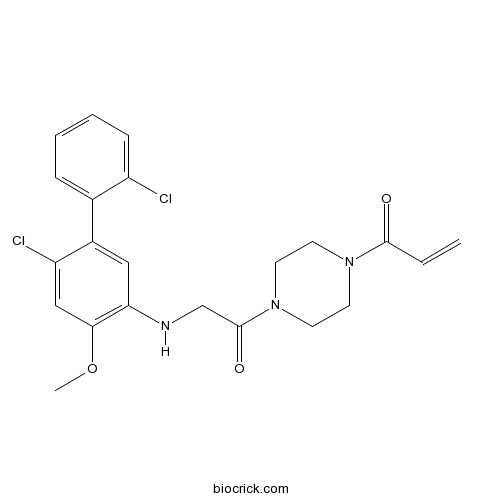
| Chemical Name | 1-[4-[2-[4-chloro-5-(2-chlorophenyl)-2-methoxyanilino]acetyl]piperazin-1-yl]prop-2-en-1-one | ||
| SMILES | COC1=C(C=C(C(=C1)Cl)C2=CC=CC=C2Cl)NCC(=O)N3CCN(CC3)C(=O)C=C | ||
| Standard InChIKey | ZJWLFLPGQCYBSE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H23Cl2N3O3/c1-3-21(28)26-8-10-27(11-9-26)22(29)14-25-19-12-16(18(24)13-20(19)30-2)15-6-4-5-7-17(15)23/h3-7,12-13,25H,1,8-11,14H2,2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

K-Ras G12C-IN-1 Dilution Calculator

K-Ras G12C-IN-1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2305 mL | 11.1523 mL | 22.3045 mL | 44.609 mL | 55.7613 mL |
| 5 mM | 0.4461 mL | 2.2305 mL | 4.4609 mL | 8.9218 mL | 11.1523 mL |
| 10 mM | 0.223 mL | 1.1152 mL | 2.2305 mL | 4.4609 mL | 5.5761 mL |
| 50 mM | 0.0446 mL | 0.223 mL | 0.4461 mL | 0.8922 mL | 1.1152 mL |
| 100 mM | 0.0223 mL | 0.1115 mL | 0.223 mL | 0.4461 mL | 0.5576 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
K-Ras G12C-IN-1 is a novel and irreversible inhibitor of mutant K-ras G12C extracted from patent WO 2014152588 A1. IC50 value: Target: K-ras G12C inhibitor
- Bis(4-bromophenyl)amine
Catalog No.:BCC8883
CAS No.:16292-17-4
- 3,4-Dihydroxybenzylamine Hydrobromide
Catalog No.:BCC8280
CAS No.:16290-26-9
- Kaempferol-7-O-D-glucopyranoside
Catalog No.:BCN2296
CAS No.:16290-07-6
- (S)-3,5-DHPG
Catalog No.:BCC6802
CAS No.:162870-29-3
- Antibiotic AB 4015B
Catalog No.:BCN1826
CAS No.:162857-79-6
- Polygalaxanthone III
Catalog No.:BCN2354
CAS No.:162857-78-5
- Polygalasaponin V
Catalog No.:BCN2790
CAS No.:162857-65-0
- 2,3,9,10-Tetrahydroxyberberine
Catalog No.:BCN3550
CAS No.:162854-37-7
- IEM 1754 dihydrobroMide
Catalog No.:BCC5049
CAS No.:162831-31-4
- 7-Epi-10-oxo-docetaxel
Catalog No.:BCC5410
CAS No.:162784-72-7
- LJI308
Catalog No.:BCC6538
CAS No.:1627709-94-7
- PFI-2
Catalog No.:BCC5561
CAS No.:1627676-59-8
- K-Ras G12C-IN-2
Catalog No.:BCC5539
CAS No.:1629267-75-9
- K-Ras G12C-IN-3
Catalog No.:BCC5540
CAS No.:1629268-19-4
- 3-O-(2-Aminoethyl)-25-hydroxyvitamin D3
Catalog No.:BCC1309
CAS No.:163018-26-6
- 2-Cl-IB-MECA
Catalog No.:BCC6938
CAS No.:163042-96-4
- Cimicifugoside H1
Catalog No.:BCN7950
CAS No.:163046-73-9
- Lup-20(29)-ene-3bate,23-diol
Catalog No.:BCN4080
CAS No.:163060-07-9
- (1R,2R)-1-Amino-2-indanol
Catalog No.:BCC8380
CAS No.:163061-73-2
- (1S,2S)-1-Amino-2-Indanol
Catalog No.:BCC8386
CAS No.:163061-74-3
- Huperzine C
Catalog No.:BCN2489
CAS No.:163089-71-2
- 17-Hydroxy-18-dehydroneogrifolin
Catalog No.:BCN7633
CAS No.:1630936-42-3
- Albatrelin G
Catalog No.:BCN7596
CAS No.:1630970-05-6
- N-Benzylmaleimide
Catalog No.:BCC9095
CAS No.:1631-26-1
Co-dependency of PKCdelta and K-Ras: inverse association with cytotoxic drug sensitivity in KRAS mutant lung cancer.[Pubmed:28368426]
Oncogene. 2017 Jul 27;36(30):4370-4378.
Recent studies suggest that the presence of a KRAS mutation may be insufficient for defining a clinically homogenous molecular group, as many KRAS mutant tumors lose reliance on K-Ras for survival. Identifying pathways that support K-Ras dependency may define clinically relevant KRAS subgroups and lead to the identification of new drug targets. We have analyzed a panel of 17 KRAS mutant lung cancer cell lines classified as K-Ras-dependent or -independent for co-dependency on protein kinase C delta (PKCdelta). We show that functional dependency on K-Ras and PKCdelta co-segregate, and that dependency correlates with a more epithelial-like phenotype. Furthermore, we show that the pro-apoptotic and pro-tumorigenic functions of PKCdelta also segregate based on K-Ras dependency, as K-Ras-independent cells are more sensitive to topoisomerase inhibitors, and depletion of PKCdelta in this subgroup suppresses apoptosis through increased activation of extracellular signal-regulated kinase (ERK). In contrast, K-Ras-dependent lung cancer cells are largely insensitive to topoisomerase inhibitors, and depletion of PKCdelta can increase apoptosis and decrease activation of ERK in this subgroup. We have previously shown that nuclear translocation of PKCdelta is necessary and sufficient for pro-apoptotic signaling. Our current studies show that K-Ras-dependent cells are refractive to PKCdelta-driven apoptosis. Analysis of this subgroup showed increased PKCdelta expression and an increase in the nuclear:cytoplasmic ratio of PKCdelta. In addition, targeting PKCdelta to the nucleus induces apoptosis in K-Ras-independent, but not K-Ras-dependent non-small-cell lung cancer (NSCLC) cells. Our studies provide tools for identification of the subset of patients with KRAS mutant tumors most amenable to targeting of the K-Ras pathway, and identify PKCdelta as a potential target in this tumor population. These subgroups are likely to be of clinical relevance, as high PKCdelta expression correlates with increased overall survival and a more epithelial tumor phenotype in patients with KRAS mutant lung adenocarcinomas.
siRNA-Encapsulated Hybrid Nanoparticles Target Mutant K-ras and Inhibit Metastatic Tumor Burden in a Mouse Model of Lung Cancer.[Pubmed:28325292]
Mol Ther Nucleic Acids. 2017 Mar 17;6:259-268.
There is an unmet need in the development of an effective therapy for mutant K-ras-expressing non-small-cell lung cancer (NSCLC). Although various small molecules have been evaluated, an effective therapy remains a dream. siRNAs have the potential to downregulate mutant K-ras both at the protein and mRNA levels. However, a safe and effective delivery of siRNAs to tumors remains a limitation to their translational application in the treatment of this highly debilitating disease. Here we developed a novel hybrid nanoparticle carrier for effective delivery of anti-mutant K-ras to NSCLC (AKSLHN). The ability of this treatment modality to regress lung tumors in mouse models was evaluated as a monotherapy or as a combination treatment with erlotinib. Further, the toxicity of this treatment modality to healthy tissues was evaluated, along with its ability to elicit immune/inflammatory reactions. The results suggest that this treatment modality is a promising prospect for the treatment of mutant K-ras-expressing NSCLC without any accompanying toxicity. However, further understanding of the cellular-level interaction between AHSLHN and erlotinib needs to be attained before this promising treatment modality can be brought to the bedside.
No back seat for a progression event-K-RAS as a therapeutic target in CRC.[Pubmed:28314765]
Genes Dev. 2017 Feb 15;31(4):333-335.
KRAS is the most frequently mutated oncogene in human cancer and plays a central, although poorly understood, role in colorectal cancer (CRC) progression. In this issue of Genes & Development, Boutin and colleagues (pp. 370-382) present a new mouse model of CRC in which the expression of oncogenic K-RAS is regulated by doxycycline. Using this model, they demonstrate that continued expression of oncogenic K-RAS is required for the survival of primary and metastatic colon cancers and that oncogenic K-RAS activates TGF-beta signaling to promote tumor invasion and metastasis.
Clinicopathological and prognostic features of surgically resected pathological stage I lung adenocarcinoma harboring epidermal growth factor receptor and K-ras mutation.[Pubmed:28322512]
Thorac Cancer. 2017 May;8(3):229-237.
BACKGROUND: This study aimed to evaluate mutations of the epidermal growth factor receptor (EGFR) and K-ras genes and their clinicopathological and prognostic features in patients with resected pathological stage I adenocarcinoma. METHODS: We examined 224 patients with surgically resected lung adenocarcinoma and analyzed the prognostic and predictive value of these mutations in 162 patients with pathological stage I adenocarcinoma. RESULTS: Mutations of the EGFR and K-ras genes were detected in 100 (44.6%) and 19 (8.5%) of all tumors, and in 81 (50.0%) and 17 (10.5%) of the pathological stage I tumors, respectively. EGFR mutations were significantly associated with female gender, smoking habit (never smoker), and low grade. By contrast, K-ras mutations were significantly associated with male gender, smoking habit (ever smoker), and the presence of mucinous components. No significant differences were observed in recurrence-free or overall survival between the EGFR-mutant, K-ras-mutant, and wild-type groups (five-year recurrence-free survival 77.8% vs. 87.8% vs. 79.5%; five-year overall survival 82.8% vs. 82.4% vs. 79.2%, respectively). Multivariate analysis showed that neither EGFR nor K-ras mutation was an independent prognostic factor. CONCLUSIONS: The present study demonstrated that pathological stage I adenocarcinoma harboring EGFR and K-ras gene mutations have distinct clinicopathological features. The presence of these mutations alone were not prognostic factors in patients with resected pathological stage I adenocarcinoma.


