KS 176Selective BCRP inhibitor CAS# 1253452-78-6 |
- Elacridar
Catalog No.:BCC1546
CAS No.:143664-11-3
- Elacridar hydrochloride
Catalog No.:BCC1547
CAS No.:143851-98-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1253452-78-6 | SDF | Download SDF |
| PubChem ID | 49779726 | Appearance | Powder |
| Formula | C22H19N3O5 | M.Wt | 405.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 35 mg/mL (86.33 mM) *"≥" means soluble, but saturation unknown. | ||
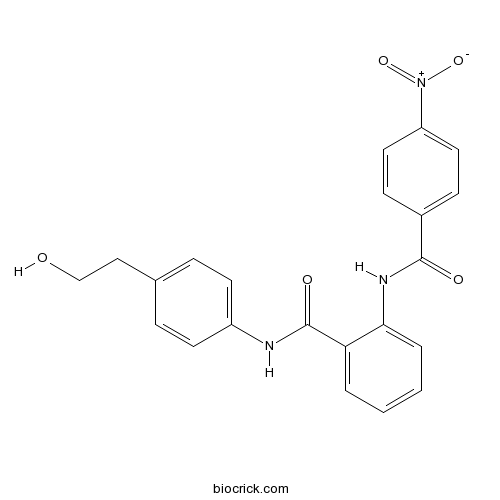
| Chemical Name | N-[4-(2-hydroxyethyl)phenyl]-2-[(4-nitrobenzoyl)amino]benzamide | ||
| SMILES | C1=CC=C(C(=C1)C(=O)NC2=CC=C(C=C2)CCO)NC(=O)C3=CC=C(C=C3)[N+](=O)[O-] | ||
| Standard InChIKey | LTWQQWSXYYXVGA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H19N3O5/c26-14-13-15-5-9-17(10-6-15)23-22(28)19-3-1-2-4-20(19)24-21(27)16-7-11-18(12-8-16)25(29)30/h1-12,26H,13-14H2,(H,23,28)(H,24,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of the breast cancer resistance protein (BCRP) multidrug transporter (IC50 values are 0.59 and 1.39 μM in Pheo A and Hoechst 33342 assays respectively). Displays no inhibitory activity against P-gp or MRP1. |

KS 176 Dilution Calculator

KS 176 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4667 mL | 12.3335 mL | 24.667 mL | 49.334 mL | 61.6675 mL |
| 5 mM | 0.4933 mL | 2.4667 mL | 4.9334 mL | 9.8668 mL | 12.3335 mL |
| 10 mM | 0.2467 mL | 1.2333 mL | 2.4667 mL | 4.9334 mL | 6.1667 mL |
| 50 mM | 0.0493 mL | 0.2467 mL | 0.4933 mL | 0.9867 mL | 1.2333 mL |
| 100 mM | 0.0247 mL | 0.1233 mL | 0.2467 mL | 0.4933 mL | 0.6167 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
KS176 is a potent and selective inhibitor of the breast cancer resistance protein (BCRP) multidrug transporter (IC50 values are 0.59 and 1.39 μM in Pheo A and Hoechst 33342 assays respectively). Displays no inhibitory activity against P-gp or MRP1. IC50 value: 0.59( in Pheo A assay), 1.39 μM (in Hoechst 33342 assay). Target: BCRP The more detailed information please refer to Compound 9 in the reference.
References:
[1]. Pick A, et al. Specific inhibitors of the breast cancer resistance protein (BCRP). ChemMedChem. 2010 Sep 3;5(9):1498-505.
- Periplogenin 3-[O-beta-glucopyranosyl-(1->4)-beta-sarmentopyranoside]
Catalog No.:BCN7861
CAS No.:1253421-94-1
- Pachysamine M
Catalog No.:BCN7309
CAS No.:1253202-75-3
- Vinorelbine Tartrate
Catalog No.:BCN2288
CAS No.:125317-39-7
- CD 437
Catalog No.:BCC7110
CAS No.:125316-60-1
- Ro 31-8220
Catalog No.:BCC4295
CAS No.:125314-64-9
- Chlorantholide D
Catalog No.:BCN4825
CAS No.:1253106-58-9
- 21,24-Epoxycycloartane-3,25-diol
Catalog No.:BCN4718
CAS No.:125305-73-9
- Wallichinine
Catalog No.:BCN6602
CAS No.:125292-97-9
- 2,4,6-Trimethoxyphenol 1-O-beta-D-glucopyranoside
Catalog No.:BCN1593
CAS No.:125288-25-7
- Kihadanin A
Catalog No.:BCN3440
CAS No.:125276-62-2
- Spiranthesol
Catalog No.:BCN7915
CAS No.:125263-69-6
- Rubiprasin B
Catalog No.:BCN7137
CAS No.:125263-66-3
- N-Debenzoyl-N-(tert-butoxycarbonyl)taxol
Catalog No.:BCN1592
CAS No.:125354-16-7
- Antiquorin
Catalog No.:BCN7163
CAS No.:125356-08-3
- NMS-E973
Catalog No.:BCC5335
CAS No.:1253584-84-7
- AbK
Catalog No.:BCC8011
CAS No.:1253643-88-7
- 6-Acetonyl-N-methyl-dihydrodecarine
Catalog No.:BCN6134
CAS No.:1253740-09-8
- Cedrelone
Catalog No.:BCN6135
CAS No.:1254-85-9
- TCN 238
Catalog No.:BCC7901
CAS No.:125404-04-8
- Acetate gossypol
Catalog No.:BCN5354
CAS No.:12542-36-8
- RQ-00203078
Catalog No.:BCC6419
CAS No.:1254205-52-1
- LY2874455
Catalog No.:BCC1723
CAS No.:1254473-64-7
- Saclofen
Catalog No.:BCC6580
CAS No.:125464-42-8
- Sibutramine hydrochloride monohydrate
Catalog No.:BCC5251
CAS No.:125494-59-9
The biological activity of nonsteroidal vitamin D hormone analogs lacking both the C- and D-rings.[Pubmed:9556055]
J Bone Miner Res. 1998 Apr;13(4):549-58.
1alpha,25-dihydroxyvitamin D is a key calcium-regulating hormone but also displays potent differentiating and antiproliferative activities on many cell types. The structural requirements of this secosteroid hormone have been extensively studied for the A-ring and side chain, whereas relatively little is known about the requirements of the natural CD-ring structure for the vitamin D-like biological activity. We have embarked on a vast program in which derivatives were synthesized and evaluated characterized by profound structural changes in the central C/D-region. This first series of nonsteroidal analogs consists of (1R,3S)-5-((Z,2E)-4-((1S,3S)-3-(4-hydroxy-4-methylpentyl)-1,2,2-++ +trimethylcyclopentyl)-2-butenylidene)-4-methylenecyclohexan e-1,3-diol (KS 176) and derivatives thereof. These analogs are characterized by the absence of normal C- and D-rings and by the presence of an unnatural five-membered ring which we call the E-ring. KS 176 with the otherwise natural side chain structure of 1alpha,25(OH)2D3 has between 10 and 30% of the biological activity of 1alpha,25(OH)2D3 when tested in vitro (prodifferentiating effects on HL-60 and MG-63; antiproliferating activity on MCF-7 and keratinocytes) but has minimal in vivo calcemic effects. Introduction of several side chain modifications created analogs with increased intrinsic noncalcemic biological properties, whereas their calcemic potency remains very low. These data demonstrate that the full CD-rings are not mandatory for the biological activity of 1alpha,25(OH)2D3 since they can be replaced by a new ring structure which generates an appropriate spacing of the A-seco B-rings in relation to the side chain. The biological activity of these nonsteroidal analogs probably involves a classical genomic activation since they are also active in transfection assays using an osteocalcin vitamin D responsive element coupled to a human growth hormone reporter gene.
Specific inhibitors of the breast cancer resistance protein (BCRP).[Pubmed:20632361]
ChemMedChem. 2010 Sep 3;5(9):1498-505.
A new class of specific breast cancer resistance protein (BCRP) inhibitors was identified, showing no inhibition of the ATP binding cassette (ABC) transporters P-gp and MRP1. Some of these modulators inhibit BCRP with high potency; they are only slightly less potent than Ko143 and could serve as promising lead structures for the design of novel effective BCRP inhibitors. These inhibitors are structurally related to tariquidar (XR9576) and belong to a library of multidrug-resistance modulators synthesized by our research group. The absence of the tetrahydroisoquinoline substructure appears to play a crucial role for specificity; we found that the presence of this substructure is not essential for interaction with BCRP. To determine the type of interaction between pheophorbide A and compounds with and without the tetrahydroisoquinoline substructure, various substrate pheophorbide A concentrations were used in enzyme kinetics assays. The resulting data show that these compounds share a noncompetitive-type interaction with pheophorbide A. Experiments with imatinib and pheophorbide A revealed a mixed-type interaction. The combination of imatinib and compounds with and without the tetrahydroisoquinoline substructure resulted in a positive cooperative effect, indicating that imatinib engages a binding site distinct from that of the new compounds on one side and distinct from that of pheophorbide A on the other side as well. The results of this study suggest that the category of BCRP-specific inhibitors, which includes only fumitremorgin C, Ko143 and analogues, and novobiocin needs to be extended by this new class of inhibitors, which possess three key characteristics: specificity, potency, and low toxicity.


