SaclofenSelective GABAB antagonist CAS# 125464-42-8 |
- Marinopyrrole A
Catalog No.:BCC4098
CAS No.:1227962-62-0
- ABT-199
Catalog No.:BCC3614
CAS No.:1257044-40-8
- BM-1074
Catalog No.:BCC2235
CAS No.:1391108-10-3
- HA14-1
Catalog No.:BCC3593
CAS No.:65673-63-4
- ABT-263 (Navitoclax)
Catalog No.:BCC1272
CAS No.:923564-51-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 125464-42-8 | SDF | Download SDF |
| PubChem ID | 122150 | Appearance | Powder |
| Formula | C9H12ClNO3S | M.Wt | 249.72 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water | ||
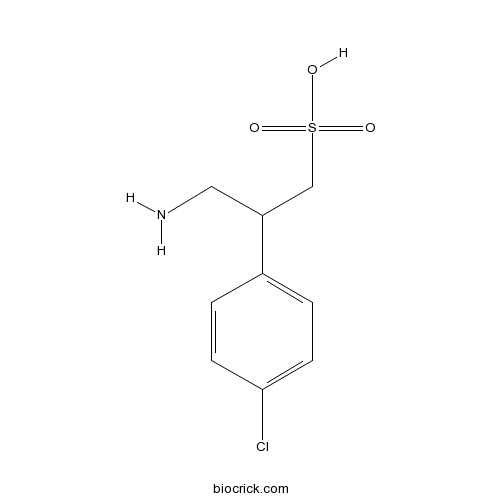
| Chemical Name | 3-amino-2-(4-chlorophenyl)propane-1-sulfonic acid | ||
| SMILES | C1=CC(=CC=C1C(CN)CS(=O)(=O)O)Cl | ||
| Standard InChIKey | JYLNVJYYQQXNEK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H12ClNO3S/c10-9-3-1-7(2-4-9)8(5-11)6-15(12,13)14/h1-4,8H,5-6,11H2,(H,12,13,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective antagonist at GABAB receptors. |

Saclofen Dilution Calculator

Saclofen Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0045 mL | 20.0224 mL | 40.0449 mL | 80.0897 mL | 100.1121 mL |
| 5 mM | 0.8009 mL | 4.0045 mL | 8.009 mL | 16.0179 mL | 20.0224 mL |
| 10 mM | 0.4004 mL | 2.0022 mL | 4.0045 mL | 8.009 mL | 10.0112 mL |
| 50 mM | 0.0801 mL | 0.4004 mL | 0.8009 mL | 1.6018 mL | 2.0022 mL |
| 100 mM | 0.04 mL | 0.2002 mL | 0.4004 mL | 0.8009 mL | 1.0011 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- LY2874455
Catalog No.:BCC1723
CAS No.:1254473-64-7
- RQ-00203078
Catalog No.:BCC6419
CAS No.:1254205-52-1
- Acetate gossypol
Catalog No.:BCN5354
CAS No.:12542-36-8
- TCN 238
Catalog No.:BCC7901
CAS No.:125404-04-8
- Cedrelone
Catalog No.:BCN6135
CAS No.:1254-85-9
- 6-Acetonyl-N-methyl-dihydrodecarine
Catalog No.:BCN6134
CAS No.:1253740-09-8
- AbK
Catalog No.:BCC8011
CAS No.:1253643-88-7
- NMS-E973
Catalog No.:BCC5335
CAS No.:1253584-84-7
- Antiquorin
Catalog No.:BCN7163
CAS No.:125356-08-3
- N-Debenzoyl-N-(tert-butoxycarbonyl)taxol
Catalog No.:BCN1592
CAS No.:125354-16-7
- KS 176
Catalog No.:BCC7874
CAS No.:1253452-78-6
- Periplogenin 3-[O-beta-glucopyranosyl-(1->4)-beta-sarmentopyranoside]
Catalog No.:BCN7861
CAS No.:1253421-94-1
- Sibutramine hydrochloride monohydrate
Catalog No.:BCC5251
CAS No.:125494-59-9
- SR 8278
Catalog No.:BCC6191
CAS No.:1254944-66-5
- Testosterone phenylpropionate
Catalog No.:BCC9171
CAS No.:1255-49-8
- Dracorhodin perchlorate
Catalog No.:BCN2628
CAS No.:125536-25-6
- 3',5,5',7-Tetrahydroxy-4',6-dimethoxyflavone
Catalog No.:BCN6136
CAS No.:125537-92-0
- LY 233053
Catalog No.:BCC5771
CAS No.:125546-04-5
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- UNC0638
Catalog No.:BCC1135
CAS No.:1255580-76-7
- MnTMPyP Pentachloride
Catalog No.:BCC6532
CAS No.:125565-45-9
- Rotigotine hydrochloride
Catalog No.:BCC1908
CAS No.:125572-93-2
- [Leu31,Pro34]-Neuropeptide Y (porcine)
Catalog No.:BCC5716
CAS No.:125580-28-1
- TRX818
Catalog No.:BCC6458
CAS No.:1256037-58-7
2-Hydroxy-saclofen causes a phaclofen-reversible reduction in population spike amplitude in the rat hippocampal slice.[Pubmed:7768279]
Eur J Pharmacol. 1995 Feb 14;274(1-3):41-6.
2-Hydroxy-Saclofen is known to be active at GABAB receptors in the mammalian central nervous system, and we have investigated its effects on synaptic transmission in the rat hippocampal slice preparation. Orthodromic stimuli were applied to the stratum radiatum, and population spike responses from the CA1 pyramidal cell layer were recorded extracellularly. A second, identical stimulus was applied at a variable interpulse interval (IPI) after the initial conditioning stimulus. GABAergic synaptic inhibition was observed as a decrease in the spike amplitude of the second response compared to the first. Both the GABAB receptor antagonist phaclofen (1 mM) and 2-hydroxy-Saclofen (200 microM) prevented a slow phase of inhibition for IPIs of 200-400 ms. However, these agents differed markedly in their effects on overall synaptic transmission. Phaclofen had no effect on the amplitude of the initial conditioning spike amplitude, whereas 2-hydroxy-Saclofen reduced it significantly, in a manner similar to baclofen (1 microM). The direct actions of 2-hydroxy-Saclofen were unexpected for a pure antagonist of GABAB receptors, but could be prevented by the co-administration of phaclofen (1 mM), but not bicuculline (1 microM). Reduction in conditioning spike amplitude due to antagonism of GABAB autoreceptors on inhibitory interneurones and subsequent enhancement of GABAA tonic inhibition would have been blocked by bicuculline. The blockade of the 2-hydroxy-Saclofen effect by phaclofen implies a GABAB receptor partial agonist action. The possible sites of this action are discussed.
Endogenous GABA release from rat striatal slices: effects of the GABAB receptor antagonist 2-hydroxy-saclofen.[Pubmed:8390105]
Synapse. 1993 May;14(1):16-23.
The reproducibility of endogenous GABA release evoked by multiple periods of electrical field stimulation was examined in rat striatal slices. In these experiments, NO-328 was used to block GABA uptake, and evoked GABA release (overflow) was completely Ca2+ dependent. A seemingly invariant observation in these experiments was that spontaneous GABA release (outflow) progressively decreased as a function of superfusion time and that GABA overflow decreased 25-30% in response to the second of two periods of stimulation (S2/S1 ratios = 0.70 to 0.75). The attenuation of GABA release was not explained by the amount of GABA lost to the superfusion buffer (fractional release), direct depletion of releasable pools of GABA, or slice viability. Furthermore, the decreases in GABA release were not dependent on stimulation frequency (5-15 Hz) or the absolute amount of GABA evoked by electrical stimulation. However, the GABAB receptor antagonist 2-hydroxy-Saclofen (2-OH-Saclofen; 316 microM) not only enhanced GABA overflow, when superfused throughout both periods of stimulation, but also resulted in S2/S1 ratios of unity. When 2-OH-Saclofen was superfused throughout the second stimulation period only, GABA overflow was almost two-fold greater than that evoked by the initial period of stimulation (2-OH-Saclofen-free). In addition, these S2 responses were approximately 30% greater than S1 responses that were observed when 2-OH-Saclofen was present throughout the entire superfusion period. These results indicate that activation of GABAB receptors was involved in the progressive attenuation of GABA release and further emphasize that GABAB receptors play an important role in modulating endogenous GABA release from striatal slices.
The human GABA(B1b) and GABA(B2) heterodimeric recombinant receptor shows low sensitivity to phaclofen and saclofen.[Pubmed:11082110]
Br J Pharmacol. 2000 Nov;131(6):1050-4.
1. The aim of this study was to characterize the pharmacological profile of the GABA(B1)/GABA(B2) heterodimeric receptor expressed in Chinese hamster ovary (CHO) cells. We have compared receptor binding affinity and functional activity for a series of agonists and antagonists. 2. The chimeric G-protein, G(qi5), was used to couple receptor activation to increases in intracellular calcium for functional studies on the Fluorimetric Imaging Plate Reader (FLIPR), using a stable GABA(B1)/GABA(B2)/G(qi5) CHO cell line. [(3)H]-CGP-54626 was used in radioligand binding studies in membranes prepared from the same cell line. 3. The pharmacological profile of the recombinant GABA(B1/B2) receptor was consistent with that of native GABA(B) receptors in that it was activated by GABA and baclofen and inhibited by CGP-54626A and SCH 50911. 4. Unlike native receptors, the GABA(B1)/GABA(B2)/G(qi5) response was not inhibited by high microMolar concentration of phaclofen, Saclofen or CGP 35348. 5. This raises the possibility that the GABA(B1)/GABA(B2)/G(qi5) recombinant receptor may represent the previously described GABA(B) receptor subtype which is relatively resistant to inhibition by phaclofen.
GABAB receptor antagonism by resolved (R)-saclofen in the guinea-pig ileum.[Pubmed:8858312]
Eur J Pharmacol. 1996 Jul 25;308(3):R1-2.
The GABAB receptor antagonist Saclofen (3-amino-2-(4-chlorophenyl)propylsulphonic acid) has been resolved by chiral high-performance liquid chromatography. The enantiomer (R)-Saclofen, but not (S)-Saclofen, reversibly antagonised the (R,S)-baclofen-induced depression of cholinergic twitch contractions in the guinea-pig ileum with an apparent pA2 of 5.3. Also, 2-hydroxy-Saclofen was resolved by the same method, its (S)-enantiomer yielding an apparent pA2 of 5.0. This method provides a convenient resolution of these antagonists.
GABAB receptors and their significance in mammalian pharmacology.[Pubmed:2559518]
Trends Pharmacol Sci. 1989 Oct;10(10):401-7.
Much progress has been made in GABAB receptor pharmacology since the discovery of this receptor in 1980. Selective agonists and antagonists have been developed and a functional role for the receptor as a mediator of slow inhibitory postsynaptic potentials in many brain regions has emerged. In this article, Norman Bowery discusses the evidence for heterogeneity of GABAB receptors, their possible physiological and pathological roles and the therapeutic potential of GABAB receptor agonists and antagonists.


