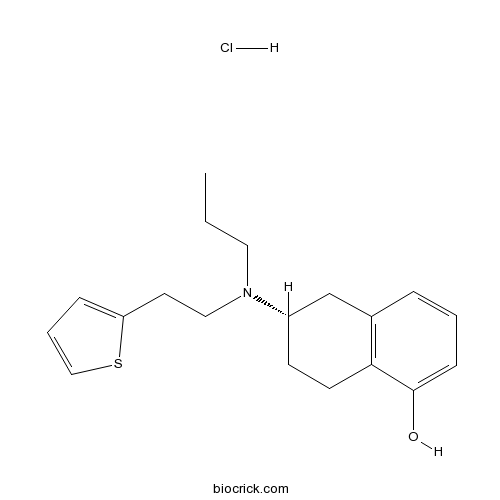
Rotigotine hydrochlorideAgonist of dopamine D2/D3 receptor CAS# 125572-93-2 |
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 125572-93-2 | SDF | Download SDF |
| PubChem ID | 180335 | Appearance | Powder |
| Formula | C19H26ClNOS | M.Wt | 351.93 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | N 0923 | ||
| Solubility | DMSO : ≥ 50 mg/mL (142.07 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (6S)-6-[propyl(2-thiophen-2-ylethyl)amino]-5,6,7,8-tetrahydronaphthalen-1-ol;hydrochloride | ||
| SMILES | CCCN(CCC1=CC=CS1)C2CCC3=C(C2)C=CC=C3O.Cl | ||
| Standard InChIKey | CEXBONHIOKGWNU-NTISSMGPSA-N | ||
| Standard InChI | InChI=1S/C19H25NOS.ClH/c1-2-11-20(12-10-17-6-4-13-22-17)16-8-9-18-15(14-16)5-3-7-19(18)21;/h3-7,13,16,21H,2,8-12,14H2,1H3;1H/t16-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dopamine D2 and D3 receptor agonist. Ki values are 13 and 0.71 nM for D2 and D3 respectively. Also has significant affinity for 5-HT1A and adrenergic α2B receptors. Exhibits antiparkinsonian acitivity. |

Rotigotine hydrochloride Dilution Calculator

Rotigotine hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8415 mL | 14.2074 mL | 28.4147 mL | 56.8295 mL | 71.0369 mL |
| 5 mM | 0.5683 mL | 2.8415 mL | 5.6829 mL | 11.3659 mL | 14.2074 mL |
| 10 mM | 0.2841 mL | 1.4207 mL | 2.8415 mL | 5.6829 mL | 7.1037 mL |
| 50 mM | 0.0568 mL | 0.2841 mL | 0.5683 mL | 1.1366 mL | 1.4207 mL |
| 100 mM | 0.0284 mL | 0.1421 mL | 0.2841 mL | 0.5683 mL | 0.7104 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Rotigotine is dopamine D2 and D3 receptor agonist. Ki values are 13 and 0.71 nM for D2 and D3 respectively. Rotigotine also has significant affinity for 5-HT1A and adrenergic α2B receptors. Rotigotine exhibits antiparkinsonian acitivity.
- MnTMPyP Pentachloride
Catalog No.:BCC6532
CAS No.:125565-45-9
- UNC0638
Catalog No.:BCC1135
CAS No.:1255580-76-7
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- LY 233053
Catalog No.:BCC5771
CAS No.:125546-04-5
- 3',5,5',7-Tetrahydroxy-4',6-dimethoxyflavone
Catalog No.:BCN6136
CAS No.:125537-92-0
- Dracorhodin perchlorate
Catalog No.:BCN2628
CAS No.:125536-25-6
- Testosterone phenylpropionate
Catalog No.:BCC9171
CAS No.:1255-49-8
- SR 8278
Catalog No.:BCC6191
CAS No.:1254944-66-5
- Sibutramine hydrochloride monohydrate
Catalog No.:BCC5251
CAS No.:125494-59-9
- Saclofen
Catalog No.:BCC6580
CAS No.:125464-42-8
- LY2874455
Catalog No.:BCC1723
CAS No.:1254473-64-7
- RQ-00203078
Catalog No.:BCC6419
CAS No.:1254205-52-1
- [Leu31,Pro34]-Neuropeptide Y (porcine)
Catalog No.:BCC5716
CAS No.:125580-28-1
- TRX818
Catalog No.:BCC6458
CAS No.:1256037-58-7
- AI-10-49
Catalog No.:BCC3973
CAS No.:1256094-72-0
- RuBi-Nicotine
Catalog No.:BCC7793
CAS No.:1256362-30-7
- Ledipasvir
Catalog No.:BCC1696
CAS No.:1256388-51-8
- CH5424802
Catalog No.:BCC3749
CAS No.:1256580-46-7
- Blinin
Catalog No.:BCN8455
CAS No.:125675-09-4
- 3''-O-acetyl-platyconic acid A
Catalog No.:BCN3319
CAS No.:1256935-28-0
- 2''-O-acetyl-platyconic acid A
Catalog No.:BCN3318
CAS No.:1256935-30-4
- CDK4 inhibitor
Catalog No.:BCC4242
CAS No.:1256963-02-6
- Lavendustin B
Catalog No.:BCN1809
CAS No.:125697-91-8
- Lavendustin A
Catalog No.:BCN1808
CAS No.:125697-92-9
Gateways to clinical trials.[Pubmed:20383346]
Methods Find Exp Clin Pharmacol. 2010 Jan-Feb;32(1):47-86.
(-)-Epigallocatechin gallate, Abafungin, ACE-031, Adapalene/benzoyl peroxide, AE-37, Aflibercept, AGS-003, Albiglutide, Alemtuzumab, Aliskiren fumarate, ALT-801, AN-2728, Anacetrapib, API, Aprepitant, ARQ-197, Ascorbic acid, Atazanavir sulfate, ATN-224, AVI-4658, Azacitidine, Azelnidipine; Belinostat, Bevacizumab, BI-2536, Biphasic insulin aspart, Bortezomib, Bovine lactoferrin, Bryostatin 1, Budesonide/formoterol fumarate; cAC10, Canfosfamide hydrochloride, Cediranib, Clofarabine, Cocaine conjugate vaccine; Darbepoetin alfa, Dasatinib, Denosumab, Disomotide, Doripenem, Dovitinib Lactate, Dronedarone hydrochloride, Drospirenone/estradiol, Dutasteride; Ecogramostim, Entinostat, Enzastaurin hydrochloride, Erlotinib hydrochloride, Everolimus, Exenatide, Ezetimibe, Ezetimibe/simvastatin; Fampridine, Fenretinide LXS, FFR-factor VIIa, Fingolimod hydrochloride, Frovatriptan; Gefitinib, Gimatecan, GP-2/GM-CSF; Iloperidone, Imatinib mesylate, Indibulin, Ipilimumab, Ivabradine hydrochloride; Lactobacillus rhamnosus, Lapatinib ditosylate, LC-07, Lenalidomide, Linifanib, Liposomal doxorubicin, Liposomal vincristine, Litenimod, Lutein; M-118, MDX-1401, MEDI-528, Midostaurin, Miglustat, MK-0657; Natalizumab, Nesiritide, NGR-TNF, Niacin/simvastatin; Obatoclax mesylate, Olaparib, Omacetaxine mepesuccinate; Paclitaxel nanoparticles, Paclitaxel-eluting stent, Palonosetron hydrochloride, Pazopanib hydrochloride, Pegfilgrastim, Pemetrexed disodium, PER.C-flu, Perifosine, PF-02341066, Pimecrolimus, Pitrakinra, Plerixafor hydrochloride, Posaconazole; Rasburicase, Recombinant human relaxin H2, ReoT3D, Retaspimycin hydrochloride, Riferminogene pecaplasmid, Rindopepimut, Romiplostim, Ronacaleret hydrochloride, Rosuvastatin calcium, Rotigotine; Sagopilone, sALP-FcD10, SAR-245409, SCH-697243, Selumetinib, Sirolimus-eluting stent, SIR-Spheres, Sitagliptin phosphate monohydrate, Sitaxentan sodium, Sorafenib, Sunitinib malate; Tadalafil, Tandutinib, Tasimelteon, Temsirolimus, Teriparatide, Tiotropium bromide, TIV, Trabectedin, Tremelimumab, TRU-016; Vadimezan, Val8-GLP-1(7-37)OH, Vandetanib, Vernakalant hydrochloride, Voreloxin, Voriconazole, Vorinostat, Yttrium 90 (90Y) ibritumomab tiuxetan; Zeaxanthin, Ziprasidone hydrochloride, Zosuquidar trihydrochloride.
Gateways to clinical trials.[Pubmed:20448862]
Methods Find Exp Clin Pharmacol. 2010 Apr;32(3):193-215.
Adefovir dipivoxil, Alemtuzumab, Aliskiren fumarate, AMA1-C1/alhydrogel, Amlodipine besylate/atorvastatin calcium, Aripiprazole, Artesunate/amodiaquine, Asenapine maleate; Bosentan, Brivaracetam; Carisbamate, Clevudine, Clofarabine, Corticorelin acetate; Dasatinib; Elinogrel potassium, Entecavir, Erlotinib hydrochloride, Eslicarbazepine acetate, Etazolate; Fampridine, Fluarix, Fondaparinux sodium, Fulvestrant; Gabapentin enacarbil, GDC-0941, GI-5005, Golimumab; Imatinib mesylate, Lacosamide, Lapatinib ditosylate, Levetiracetam, Liraglutide, LOLA; Mecasermin, Morphine hydrochloride; Natalizumab, Nilotinib hydrochloride monohydrate; Olmesartan medoxomil, Omacetaxine mepesuccinate; Paclitaxel-eluting stent, Peginterferon alfa-2a, Peginterferon alfa-2b, Pemetrexed disodium, Poly I:CLC, Pralatrexate, Pregabalin; Ranolazine, Rasagiline mesilate, Retigabine hydrochloride, Rhenium Re-186 etidronate, Rosuvastatin calcium, Rotigotine, RTL-1000, Rufinamide; Sirolimus-eluting coronary stent, Sirolimus-eluting stent, Sorafenib, Stiripentol; Tiotropium bromide; Valsartan/amlodipine besylate, Varenicline tartrate; XL-184; Zoledronic acid monohydrate.
In vivo dopamine agonist properties of rotigotine: Role of D1 and D2 receptors.[Pubmed:27343381]
Eur J Pharmacol. 2016 Oct 5;788:183-191.
Rotigotine acts in vitro as a full agonist of dopamine D1 receptors at concentrations almost superimposable to those at which it acts on D2 receptors. However in vivo evidence of the differences between the agonist activity of rotigotine at D1 receptors from that on the D2 receptors has not been provided yet. In order to test the ability of rotigotine to stimulate dopamine D1 and D2 receptors in vivo, we studied the effect of SCH39166 and eticlopride, selective dopamine D1 and D2/D3 receptor antagonists respectively, on rotigotine-induced contralateral turning behavior in 6-hydroxydopamine lesioned rats. Furthermore, the expression of the immediate-early gene c-fos in the caudate-putamen, was evaluated. As a comparison, we tested the D2/D3 agonist pramipexole. In primed rats, rotigotine (0.035, 0.1 and 0.35mg/kg) induced dose-dependent contralateral turning. Turning induced by 0.1mg/kg of rotigotine was reduced by pretreatment with the D1 antagonist SCH39166 and the D2 antagonist eticlopride. In drug-naive rats, rotigotine was less effective in eliciting turning but SCH39166 still reduced turning induced by rotigotine (0.35mg/kg). Pramipexole induced contralateral turning only in primed rats. SCH39166 potentiated and eticlopride abolished pramipexole-induced turning. Rotigotine induced Fos expression in the caudate-putamen and SCH39166 completely blocked it. Pramipexole failed to induce Fos. These results indicate that rotigotine acts in vivo as an agonist of D1 and D2 receptors while pramipexole is devoid of D1 activity in vivo. Given their differing DA receptor profiles, rotigotine and pramipexole might differ in their spectrum of application to the therapy of Parkinson's disease.
New functional activity of aripiprazole revealed: Robust antagonism of D2 dopamine receptor-stimulated Gbetagamma signaling.[Pubmed:25449598]
Biochem Pharmacol. 2015 Jan 1;93(1):85-91.
The dopamine D2 receptor (DRD2) is a G protein-coupled receptor (GPCR) that is generally considered to be a primary target in the treatment of schizophrenia. First generation antipsychotic drugs (e.g. haloperidol) are antagonists of the DRD2, while second generation antipsychotic drugs (e.g. olanzapine) antagonize DRD2 and 5HT2A receptors. Notably, both these classes of drugs may cause side effects associated with D2 receptor antagonism (e.g. hyperprolactemia and extrapyramidal symptoms). The novel, "third generation" antipsychotic drug, aripiprazole is also used to treat schizophrenia, with the remarkable advantage that its tendency to cause extrapyramidal symptoms is minimal. Aripiprazole is considered a partial agonist of the DRD2, but it also has partial agonist/antagonist activity for other GPCRs. Further, aripiprazole has been reported to have a unique activity profile in functional assays with the DRD2. In the present study the molecular pharmacology of aripiprazole was further examined in HEK cell models stably expressing the DRD2 and specific isoforms of adenylyl cyclase to assess functional responses of Galpha and Gbetagamma subunits. Additional studies examined the activity of aripiprazole in DRD2-mediated heterologous sensitization of adenylyl cyclase and cell-based dynamic mass redistribution (DMR). Aripiprazole displayed a unique functional profile for modulation of G proteins, being a partial agonist for Galphai/o and a robust antagonist for Gbetagamma signaling. Additionally, aripiprazole was a weak partial agonist for both heterologous sensitization and dynamic mass redistribution.
Gateways to clinical trials.[Pubmed:20508873]
Methods Find Exp Clin Pharmacol. 2010 May;32(4):247-88.
O(6)-Benzylguanine; (-)-Gossypol; Abatacept, AC-2592, Adalimumab, AIDSVAX gp120 B/E, Alemtuzumab, Aliskiren fumarate, ALVAC E120TMG, Ambrisentan, Amlodipine, Anakinra, Aripiprazole, Armodafinil, Atomoxetine hydrochloride, Avotermin; Bevacizumab, BIBW-2992, Bortezomib, Bosentan, Botulinum toxin type B; Canakinumab, CAT-354, Ciclesonide, CMV gB vaccine, Corifollitropin alfa, Daptomycin, Darbepoetin alfa, Dasatinib, Denosumab; EndoTAG-1, Eplerenone, Esomeprazole sodium, Eszopiclone, Etoricoxib, Everolimus, Exenatide, Ezetimibe, Ezetimibe/simvastatin; F-50040, Fesoterodine fumavate, Fondaparinux sodium, Fulvestrant; Gabapentin enacarbil, Golimumab; Imatinib mesylate, Inhalable human insulin, Insulin glargine, Ivabradine hydrochloride; Lercanidipine hydrochloride/enalapril maleate, Levosimendan, Liposomal vincristine sulfate, Liraglutide; MDV-3100, Mometasone furoate/formoterol fumavate, Multiepitope CTL peptide vaccine, Mycophenolic acid sodium salt, Nabiximols, Natalizumab, Nesiritide; Obeticholic acid, Olmesartan medoxomil, Omalizumab, Omecamtiv mecarbil; Paclitaxel-eluting stent, Paliperidone, Pegfilgrastim, Peginterferon alfa-2a, Peginterferon alfa-2b, Peginterferon alfa-2b/ ribavirin, Pemetrexed disodium, Polymyxin B nonapeptide, PORxin-302, Prasugrel, Pregabalin, Pridopidine; Ranelic acid distrontium salt, Rasagiline mesilate, rDEN4delta30-4995, Recombinant human relaxin H2, rhFSH, Rilonacept, Rolofylline, Rosiglitazone maleate/metformin hydrochloride, Rosuvastatin calcium, Rotigotine; Salcaprozic acid sodium salt, Sirolimus-eluting stent, Sitagliptin phosphate monohydrate, Sitaxentan sodium, Sorafenib, Sunitinib malate; Tadalafil, Tapentadol hydrochloride, Temsirolimus, Tenofovir, Tenofovir disoproxil fumarate, Teriparatide, Tiotropium bromide, Tocilizumab, Tolvaptan, Tozasertib, Treprostinil sodium; Ustekinumab; Vardenafil hydrochloride hydrate, Varenicline tartrate, Vatalanib succinate, Voriconazole, Vorinostat; Zotarolimus-eluting stent.
The in vitro receptor profile of rotigotine: a new agent for the treatment of Parkinson's disease.[Pubmed:18704368]
Naunyn Schmiedebergs Arch Pharmacol. 2009 Jan;379(1):73-86.
Rotigotine (Neupro) is a non-ergoline dopamine agonist developed for the once daily treatment of Parkinson's disease (PD) using a transdermal delivery system (patch) which provides patients with the drug continuously over 24 h. To fully understand the pharmacological actions of rotigotine, the present study determined its extended receptor profile. In standard binding assays, rotigotine demonstrated the highest affinity for dopamine receptors, particularly the dopamine D3 receptor (Ki=0.71 nM) with its affinities to other dopamine receptors being (Ki in nM): D4.2 (3.9), D4.7 (5.9), D5 (5.4), D2 (13.5), D4.4 (15), and D1 (83). Significant affinities were also demonstrated at alpha-adrenergic (alpha2B, Ki=27 nM) and serotonin receptors (5-HT1A Ki=30 nM). In newly developed reporter-gene assays for determination of functional activity, rotigotine behaved as a full agonist at dopamine receptors (rank order: D3>D2L>D1=D5>D4.4) with potencies 2,600 and 53 times higher than dopamine at dopamine D3 and D2L receptors, respectively. At alpha-adrenergic sites, rotigotine acted as an antagonist on alpha2B receptors. At serotonergic sites, rotigotine had a weak but significant agonistic activity at 5-HT1A receptors and a minor or nonexistent activity at other serotonin receptors. Thus, in respect to PD, rotigotine can be characterized as a specific dopamine receptor agonist with a preference for the D3 receptor over D2 and D1 receptors. In addition, it exhibits interaction with D4 and D5 receptors, the role of which in relation to PD is not clear yet. Among non-dopaminergic sites, rotigotine shows relevant affinity to only 5-HT1A and alpha2B receptors. Further studies are necessary to investigate the contribution of the different receptor subtypes to the efficacy of rotigotine in Parkinson's disease and possible other indications such as restless legs syndrome.


