AbKCAS# 1253643-88-7 |
- PF-4981517
Catalog No.:BCC2270
CAS No.:1390637-82-7
- VT-464
Catalog No.:BCC5398
CAS No.:1610537-15-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1253643-88-7 | SDF | Download SDF |
| PubChem ID | 66825403 | Appearance | Powder |
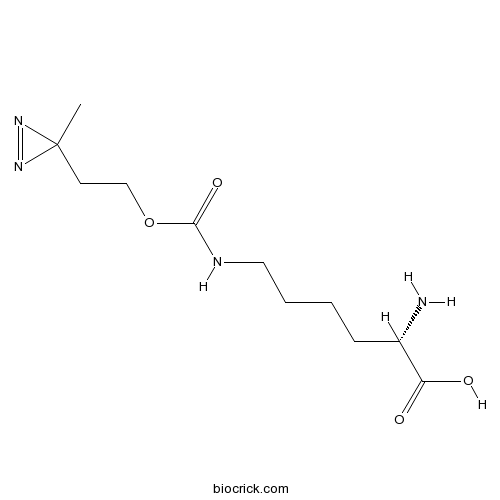
| Formula | C11H20N4O4 | M.Wt | 272.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water with gentle warming and to 100 mM in 1eq. HCl | ||
| Chemical Name | (2S)-2-amino-6-[2-(3-methyldiazirin-3-yl)ethoxycarbonylamino]hexanoic acid | ||
| SMILES | CC1(N=N1)CCOC(=O)NCCCCC(C(=O)O)N | ||
| Standard InChIKey | LUCMNTLJAFTFDU-QMMMGPOBSA-N | ||
| Standard InChI | InChI=1S/C11H20N4O4/c1-11(14-15-11)5-7-19-10(18)13-6-3-2-4-8(12)9(16)17/h8H,2-7,12H2,1H3,(H,13,18)(H,16,17)/t8-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Unnatural amino acid. Acts as a UV light-activated photo-crosslinking probe when incorporated into proteins by pyrrolysyl tRNA synthetase/tRNACUA pairs. |

AbK Dilution Calculator

AbK Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6724 mL | 18.3621 mL | 36.7242 mL | 73.4484 mL | 91.8105 mL |
| 5 mM | 0.7345 mL | 3.6724 mL | 7.3448 mL | 14.6897 mL | 18.3621 mL |
| 10 mM | 0.3672 mL | 1.8362 mL | 3.6724 mL | 7.3448 mL | 9.1811 mL |
| 50 mM | 0.0734 mL | 0.3672 mL | 0.7345 mL | 1.469 mL | 1.8362 mL |
| 100 mM | 0.0367 mL | 0.1836 mL | 0.3672 mL | 0.7345 mL | 0.9181 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- NMS-E973
Catalog No.:BCC5335
CAS No.:1253584-84-7
- Antiquorin
Catalog No.:BCN7163
CAS No.:125356-08-3
- N-Debenzoyl-N-(tert-butoxycarbonyl)taxol
Catalog No.:BCN1592
CAS No.:125354-16-7
- KS 176
Catalog No.:BCC7874
CAS No.:1253452-78-6
- Periplogenin 3-[O-beta-glucopyranosyl-(1->4)-beta-sarmentopyranoside]
Catalog No.:BCN7861
CAS No.:1253421-94-1
- Pachysamine M
Catalog No.:BCN7309
CAS No.:1253202-75-3
- Vinorelbine Tartrate
Catalog No.:BCN2288
CAS No.:125317-39-7
- CD 437
Catalog No.:BCC7110
CAS No.:125316-60-1
- Ro 31-8220
Catalog No.:BCC4295
CAS No.:125314-64-9
- Chlorantholide D
Catalog No.:BCN4825
CAS No.:1253106-58-9
- 21,24-Epoxycycloartane-3,25-diol
Catalog No.:BCN4718
CAS No.:125305-73-9
- Wallichinine
Catalog No.:BCN6602
CAS No.:125292-97-9
- 6-Acetonyl-N-methyl-dihydrodecarine
Catalog No.:BCN6134
CAS No.:1253740-09-8
- Cedrelone
Catalog No.:BCN6135
CAS No.:1254-85-9
- TCN 238
Catalog No.:BCC7901
CAS No.:125404-04-8
- Acetate gossypol
Catalog No.:BCN5354
CAS No.:12542-36-8
- RQ-00203078
Catalog No.:BCC6419
CAS No.:1254205-52-1
- LY2874455
Catalog No.:BCC1723
CAS No.:1254473-64-7
- Saclofen
Catalog No.:BCC6580
CAS No.:125464-42-8
- Sibutramine hydrochloride monohydrate
Catalog No.:BCC5251
CAS No.:125494-59-9
- SR 8278
Catalog No.:BCC6191
CAS No.:1254944-66-5
- Testosterone phenylpropionate
Catalog No.:BCC9171
CAS No.:1255-49-8
- Dracorhodin perchlorate
Catalog No.:BCN2628
CAS No.:125536-25-6
- 3',5,5',7-Tetrahydroxy-4',6-dimethoxyflavone
Catalog No.:BCN6136
CAS No.:125537-92-0
A possible immunomodulating activity of arbekacin (ABK), a newly synthesized antibiotic against methicillin-resistant Staphylococcus aureus (MRSA).[Pubmed:8045672]
Int J Immunopharmacol. 1994 Apr;16(4):321-7.
Arbekacin (AbK) is a newly developed aminoglycoside in Japan which can cause bactericidal effects on methicillin-resistant Staphylococcus aureus (MRSA). However, details of the mechanism on effective antibacterial activity of this drug in host patients has not been completely elucidated. We studied the modulating effects on some functional activities of immunocompetent cells in in vitro and in vivo mouse experimental systems. (i) AbK stimulated the phagocytic activity against S. aureus into mouse phagocytic cells in a dose-dependent manner. (ii) AbK enhanced oxygen radical generation with chemiluminescence in mouse phagocytic cells. (iii) AbK exhibited a stimulatory effect on interleukin 1 alpha release from mouse adherent cells. (iv) Injection with AbK into mice showed an adjuvant activity which stimulated antibody production against sheep red blood cells and S. aureus. These experimental results indicate the possibility that AbK has not only a direct antibacterial activity but also a modulating activity for immunocompetent cells to enhance host defense against S. aureus infection.
[Clinical analysis of MRSA pneumonia. Niigata Research Group of MRSA.ABK].[Pubmed:8072182]
Jpn J Antibiot. 1994 Jun;47(6):736-40.
Aged or immuno-compromised patients were mostly affected, by pneumonia caused by infection of MRSA, and more than half of the cases were superinfected with glucose-nonfermentative Gram-negative rods including Pseudomonas aeruginosa. These patients were treated with a monotherapy of arbekacin (AbK) by intravenous drip administration or with a combination of AbK and imipenem/cilastatin, ceftazidime or antifungals. The clinical efficiencies were 55.6% in 11 monotherapy cases and 83.3% in combined therapy. MRSA was eradicated in 31.9% of the patients. These results are comparable with, or superior to the vancomycin therapy in the treatment of MRSA pneumonia. When MRSA is isolated from sputum of pneumonia patients, the discrimination between colonization and infection is important, but the diagnosis is very difficult in many clinical cases before the initiation of chemotherapy. The number of bacteria and the grade of inflammation should be carefully scored before starting a chemotherapy.
Low cost single-step purification of endoglucanase from Aspergillus fumigatus ABK-9.[Pubmed:24416930]
Indian J Exp Biol. 2013 Nov;51(11):954-9.
Low cost agro-waste was used as adsorption support for single-step purification of endoglucanase from the culture filtrate of A. fumigatus AbK-9. Among various agro-waste substrates, 1% NaOH pretreated rice bran was proved to be the best for adsorbing about 74.8 and 71.1% of endoglucanase at 4 degrees C and 10 degrees C respectively. Langmuir type adsorption isotherm at 4 degrees C showed maximum adsorption of enzyme at pH 5.0, which was in the range of optimum pH of the enzyme. The rice bran column bound enzyme was maximally eluted by a mixture of acetate buffer (0.05 M, pH 5.5) and ethanol (40%, v/v) at a ratio of 3:2 and a flow rate of 1 mL/min. A 5.52-fold purification of the enzyme was achieved from culture supernatant. The specific activity and recovery yield after purification were 294.0 U/mg and 40.15%, respectively, which were comparable with other contemporary protocols. The homogeneity of the enzyme was tested through sodium dodecyl sulphate polyacrylamide gel electrophoresis and a single band of 56.3 kDa was observed. Zymogram analysis finally confirmed the occurrence of endoglucanase in the single band.
Efficient viral delivery system for unnatural amino acid mutagenesis in mammalian cells.[Pubmed:23818609]
Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):11803-8.
Here we report the development of a baculovirus-based delivery system that enables the efficient incorporation of unnatural amino acids into proteins in mammalian cells. We have exploited the large cargo-capacity (>30 kb) and stability of the double-stranded DNA genome of baculovirus to deliver to a variety of cell types all of the components required to genetically incorporate novel amino acids. These include the engineered tRNA/aminoacyl-tRNA synthetase pair and the nonsense mutant of the target gene. Mammalian cell transduction efficiency of baculovirus was significantly improved by incorporating genetic elements from mammalian viruses. Two polyspecific tRNA/aminoacyl-tRNA synthetase pairs were inserted into this expression system, enabling the site-specific incorporation of a variety of unnatural amino acids with novel chemical and biological properties into proteins.
Visualizing protein partnerships in living cells and organisms.[Pubmed:22104179]
Curr Opin Chem Biol. 2011 Dec;15(6):781-8.
In recent years, scientists have expanded their focus from cataloging genes to characterizing the multiple states of their translated products. One anticipated result is a dynamic map of the protein association networks and activities that occur within the cellular environment. While in vitro-derived network maps can illustrate which of a multitude of possible protein-protein associations could exist, they supply a falsely static picture lacking the subtleties of subcellular location (where) or cellular state (when). Generating protein association network maps that are informed by both subcellular location and cell state requires novel approaches that accurately characterize the state of protein associations in living cells and provide precise spatiotemporal resolution. In this review, we highlight recent advances in visualizing protein associations and networks under increasingly native conditions. These advances include second generation protein complementation assays (PCAs), chemical and photo-crosslinking techniques, and proximity-induced ligation approaches. The advances described focus on background reduction, signal optimization, rapid and reversible reporter assembly, decreased cytotoxicity, and minimal functional perturbation. Key breakthroughs have addressed many challenges and should expand the repertoire of tools useful for generating maps of protein interactions resolved in both time and space.


