WallichinineCAS# 125292-97-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 125292-97-9 | SDF | Download SDF |
| PubChem ID | 5315280 | Appearance | Oil |
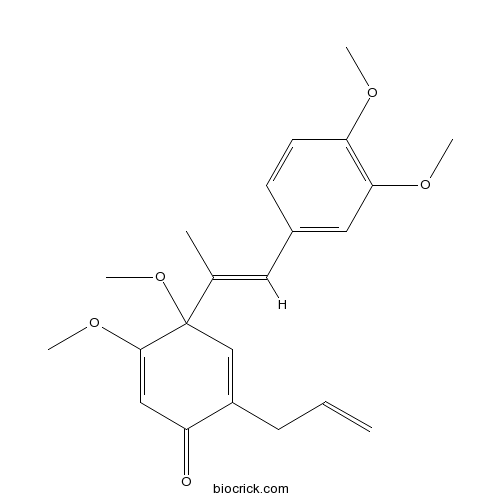
| Formula | C22H26O5 | M.Wt | 370.5 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-[(E)-1-(3,4-dimethoxyphenyl)prop-1-en-2-yl]-4,5-dimethoxy-2-prop-2-enylcyclohexa-2,5-dien-1-one | ||
| SMILES | CC(=CC1=CC(=C(C=C1)OC)OC)C2(C=C(C(=O)C=C2OC)CC=C)OC | ||
| Standard InChIKey | VKYZYTYFGUQBLS-RVDMUPIBSA-N | ||
| Standard InChI | InChI=1S/C22H26O5/c1-7-8-17-14-22(27-6,21(26-5)13-18(17)23)15(2)11-16-9-10-19(24-3)20(12-16)25-4/h7,9-14H,1,8H2,2-6H3/b15-11+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Wallichinine shows inhibitory activity on platelet aggregation caused by platelet activating factor (PAF). 2. Wallichinine can significantly potentiate the effects of two ABCB1 substrates vincristine and doxorubicin on inhibition of growth, arrest of cell cycle and induction of apoptosis in ABCB1 overexpressing cancer cells, and the overexpression of ABCB1 in cancer cells is one of the main reasons of cancer multidrug resistance (MDR), suggests that wallichinine with ABCB1 presents valuable clues for the development of novel MDR reversal reagents from natural products. |
| Targets | PAFR |

Wallichinine Dilution Calculator

Wallichinine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6991 mL | 13.4953 mL | 26.9906 mL | 53.9811 mL | 67.4764 mL |
| 5 mM | 0.5398 mL | 2.6991 mL | 5.3981 mL | 10.7962 mL | 13.4953 mL |
| 10 mM | 0.2699 mL | 1.3495 mL | 2.6991 mL | 5.3981 mL | 6.7476 mL |
| 50 mM | 0.054 mL | 0.2699 mL | 0.5398 mL | 1.0796 mL | 1.3495 mL |
| 100 mM | 0.027 mL | 0.135 mL | 0.2699 mL | 0.5398 mL | 0.6748 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2,4,6-Trimethoxyphenol 1-O-beta-D-glucopyranoside
Catalog No.:BCN1593
CAS No.:125288-25-7
- Kihadanin A
Catalog No.:BCN3440
CAS No.:125276-62-2
- Spiranthesol
Catalog No.:BCN7915
CAS No.:125263-69-6
- Rubiprasin B
Catalog No.:BCN7137
CAS No.:125263-66-3
- Rubiprasin A
Catalog No.:BCN7138
CAS No.:125263-65-2
- (1beta,3beta,25S)-3-Hydroxyspirost-5-en-1-yl 2-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-xylopyranoside
Catalog No.:BCN8164
CAS No.:125225-63-0
- Tubastatin A
Catalog No.:BCC2158
CAS No.:1252003-15-8
- 1,7-Bis(4-hydroxyphenyl)hept-6-en-3-one
Catalog No.:BCN6597
CAS No.:1251830-57-5
- Rosthornin B
Catalog No.:BCN6133
CAS No.:125181-21-7
- N-Methoxyanhydrovobasinediol
Catalog No.:BCN4856
CAS No.:125180-42-9
- Vibralactone D
Catalog No.:BCN6747
CAS No.:1251748-32-9
- Rosthornin A
Catalog No.:BCN6132
CAS No.:125164-55-8
- 21,24-Epoxycycloartane-3,25-diol
Catalog No.:BCN4718
CAS No.:125305-73-9
- Chlorantholide D
Catalog No.:BCN4825
CAS No.:1253106-58-9
- Ro 31-8220
Catalog No.:BCC4295
CAS No.:125314-64-9
- CD 437
Catalog No.:BCC7110
CAS No.:125316-60-1
- Vinorelbine Tartrate
Catalog No.:BCN2288
CAS No.:125317-39-7
- Pachysamine M
Catalog No.:BCN7309
CAS No.:1253202-75-3
- Periplogenin 3-[O-beta-glucopyranosyl-(1->4)-beta-sarmentopyranoside]
Catalog No.:BCN7861
CAS No.:1253421-94-1
- KS 176
Catalog No.:BCC7874
CAS No.:1253452-78-6
- N-Debenzoyl-N-(tert-butoxycarbonyl)taxol
Catalog No.:BCN1592
CAS No.:125354-16-7
- Antiquorin
Catalog No.:BCN7163
CAS No.:125356-08-3
- NMS-E973
Catalog No.:BCC5335
CAS No.:1253584-84-7
- AbK
Catalog No.:BCC8011
CAS No.:1253643-88-7
Wallichinine reverses ABCB1-mediated cancer multidrug resistance.[Pubmed:27508017]
Am J Transl Res. 2016 Jul 15;8(7):2969-80. eCollection 2016.
Overexpression of ABCB1 in cancer cells is one of the main reasons of cancer multidrug resistance (MDR). Wallichinine is a compound isolated from piper wallichii and works as an antagonist of platelet activiating factor receptor to inhibit the gathering of blood platelet. In this study, we investigate the effect of Wallichinine on cancer MDR mediated by ABCB1 transporter. Wallichinine significantly potentiates the effects of two ABCB1 substrates vincristine and doxorubicin on inhibition of growth, arrest of cell cycle and induction of apoptosis in ABCB1 overexpressing cancer cells. Furthermore, Wallichinine do not alter the sensitivity of non-ABCB1 substrate cisplatin. Mechanistically, Wallichinine blocks the drug-efflux activity of ABCB1 to increase the intracellular accumulation of rhodamine 123 and doxorubicin and stimulates the ATPase of ABCB1 without alteration of the expression of ABCB1. The predicted binding mode shows the hydrophobic interactions of Wallichinine within the large drug binding cavity of ABCB1. At all, our study of the interaction of Wallichinine with ABCB1 presented herein provides valuable clues for the development of novel MDR reversal reagents from natural products.
[The isolation and identification of PAF inhibitors from Piper wallichii (Miq.) Hand-Mazz and P. hancei Maxim].[Pubmed:2609983]
Yao Xue Xue Bao. 1989;24(6):438-43.
Platelet activating factor (PAF) is a highly potent endogenous phospholipid mediator, involved in various inflammatory and cardiovascular disorders. As part of a research program dealing with PAF inhibitors isolated from Piper plant species, we have isolated kadsurenone (I), denudatin B (II), and N-isobutyl-deca-trans-2-trans-4-dienamide (III) from Piped wallichii (Miq.) Hand-Mazz. and P. hancei Maxim. In a continuing search for potential PAF inhibitor from plants, using PAF induced platelet aggregation as a guide, a new neolignan named hancinone D (IV) was isolated from P. hancei maxim. By X-ray analysis it was identified as a racemate. The X-ray analysis led to a revision of the previously made structure assignment of hancinone C. Another new neolignan named Wallichinine (V), which was identified as an analogue of (IV), along with the known compounds hancinone C (VI), galgravin (VII), dihydropiperlonguminine (VIII) and crotepoxide (IX) were isolated from P. wallichii (Miq.) Hand-Mazz. The structure determination was based upon spectroscopic analysis. All of the compounds were for the first time obtained from both plants. In the test of platelet aggregation caused by PAF, I, II, V, VI, VII showed inhibitory activity, whereas III, IV, VII, IX showed no activity.


