LRGILS-NH2Control peptide for SLIGRL-NH2 CAS# 245329-01-5 |
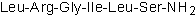
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 245329-01-5 | SDF | Download SDF |
| PubChem ID | 90488840 | Appearance | Powder |
| Formula | C29H56N10O7 | M.Wt | 656.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 2 mg/ml in 30% acetonitrile / water | ||
| Sequence | LRGILS (Modifications: Ser-6 = C-terminal amide) | ||
| Chemical Name | (2S,3S)-N-[(2S)-1-[[(2S)-1-amino-3-hydroxy-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]-2-[[2-[[(2S)-2-[[(2S)-2-amino-4-methylpentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]acetyl]amino]-3-methylpentanamide | ||
| SMILES | CCC(C)C(C(=O)NC(CC(C)C)C(=O)NC(CO)C(=O)N)NC(=O)CNC(=O)C(CCCN=C(N)N)NC(=O)C(CC(C)C)N | ||
| Standard InChIKey | HCUFWKYFOMBFPA-JYAZKYGWSA-N | ||
| Standard InChI | InChI=1S/C29H56N10O7/c1-7-17(6)23(28(46)37-20(12-16(4)5)27(45)38-21(14-40)24(31)42)39-22(41)13-35-26(44)19(9-8-10-34-29(32)33)36-25(43)18(30)11-15(2)3/h15-21,23,40H,7-14,30H2,1-6H3,(H2,31,42)(H,35,44)(H,36,43)(H,37,46)(H,38,45)(H,39,41)(H4,32,33,34)/t17-,18-,19-,20-,21-,23-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reversed amino acid sequence control peptide for SLIGRL-NH2, a protease-activated receptor-2 (PAR2) agonist that facilitates gastrointestinal transit in vivo. |

LRGILS-NH2 Dilution Calculator

LRGILS-NH2 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Reversed amino acid sequence control peptide for SLIGRL-NH2, a protease-activated receptor-2 (PAR2) agonist that facilitates gastrointestinal transit in vivo.
- Makisterone A 20,22-monoacetonide
Catalog No.:BCN7090
CAS No.:245323-24-4
- 1,2-Dioleoyl-sn-glycerol
Catalog No.:BCC6416
CAS No.:24529-88-2
- 2-Cyano-N-[4-(Trifluoromethyl)Phenyl]Acetamide
Catalog No.:BCC8571
CAS No.:24522-30-3
- n-Tritriacontan-16,18-dione
Catalog No.:BCC9106
CAS No.:24514-86-1
- 6alpha-Hydroxypolyporenic acid C
Catalog No.:BCN3647
CAS No.:24513-63-1
- 21-Episerratriol
Catalog No.:BCN5107
CAS No.:24513-57-3
- 3,21-Dihydroxy-14-serraten-16-one
Catalog No.:BCN5106
CAS No.:24513-51-7
- Monomethyl kolavate
Catalog No.:BCN5105
CAS No.:24513-41-5
- Geniposide
Catalog No.:BCN5104
CAS No.:24512-63-8
- Gardenoside
Catalog No.:BCN2383
CAS No.:24512-62-7
- 19-Oxocinobufotalin
Catalog No.:BCN8233
CAS No.:24512-60-5
- 19-Oxocinobufagin
Catalog No.:BCN8229
CAS No.:24512-59-2
- FSLLRY-NH2
Catalog No.:BCC6279
CAS No.:245329-02-6
- LED209
Catalog No.:BCC6437
CAS No.:245342-14-7
- Decursitin D
Catalog No.:BCN3908
CAS No.:245446-61-1
- (2S,3R,E)-2-Amino-4-tetradecene-1,3-diol
Catalog No.:BCN1478
CAS No.:24558-60-9
- 7,8,9-Trimethoxy-10H-1,3-dioxolo[4,5-b]xanthen-10-one
Catalog No.:BCN1477
CAS No.:24562-58-1
- 5'-S-Methyl-5'-thioadenosine
Catalog No.:BCN5108
CAS No.:2457-80-9
- (+)-Muscarine iodide
Catalog No.:BCC7556
CAS No.:24570-49-8
- RWJ 52353
Catalog No.:BCC6086
CAS No.:245744-10-9
- TC-G 1000
Catalog No.:BCC7991
CAS No.:245744-18-7
- Rubrofusarin-6-O-beta-D-gentiobioside
Catalog No.:BCN1476
CAS No.:24577-90-0
- trans-4-Hydroxycrotonic acid
Catalog No.:BCC6685
CAS No.:24587-49-3
- BIIE 0246
Catalog No.:BCC7158
CAS No.:246146-55-4
Modulation of capsaicin-evoked visceral pain and referred hyperalgesia by protease-activated receptors 1 and 2.[Pubmed:15037813]
J Pharmacol Sci. 2004 Mar;94(3):277-85.
Protease-activated receptors (PARs) 1 and 2 are expressed in capsaicin-sensitive sensory neurons, being anti- and pro-nociceptive, respectively. Given the possible cross talk between PAR-2 and capsaicin receptors, we investigated if PAR-2 activation could facilitate capsaicin-evoked visceral pain and referred hyperalgesia in the mouse and also examined the effect of PAR-1 activation in this model. Intracolonic (i.col.) administration of capsaicin triggered visceral pain-related nociceptive behavior, followed by referred hyperalgesia. The capsaicin-evoked visceral nociception was suppressed by intraperitoneal (i.p.) TFLLR-NH2, a PAR-1-activating peptide, but not FTLLR-NH2, a control peptide, and unaffected by i.col. TFLLR-NH2. SLIGRL-NH2, a PAR-2-activating peptide, but not LRGILS-NH2, a control peptide, administered i.col., facilitated the capsaicin-evoked visceral nociception 6-18 h after administration, while i.p. SLIGRL-NH2 had no effect. The capsaicin-evoked referred hyperalgesia was augmented by i.col. SLIGRL-NH2, but not LRGILS-NH2, 6-18 h after administration, and unaffected by i.p. SLIGRL-NH2, and i.p. or i.col. TFLLR-NH2. Our data suggest that PAR-1 is antinociceptive in processing of visceral pain, whereas PAR-2 expressed in the colonic luminal surface, upon activation, produces delayed sensitization of capsaicin receptors, resulting in facilitation of visceral pain and referred hyperalgesia.
Protease-activated receptor 2 activation inhibits N-type Ca2+ currents in rat peripheral sympathetic neurons.[Pubmed:25410909]
Mol Cells. 2014 Nov;37(11):804-11.
The protease-activated receptor (PAR)-2 is highly expressed in endothelial cells and vascular smooth muscle cells. It plays a crucial role in regulating blood pressure via the modulation of peripheral vascular tone. Although several mechanisms have been suggested to explain PAR-2-induced hypotension, the precise mechanism remains to be elucidated. To investigate this possibility, we investigated the effects of PAR-2 activation on N-type Ca(2+) currents (I(Ca-N)) in isolated neurons of the celiac ganglion (CG), which is involved in the sympathetic regulation of mesenteric artery vascular tone. PAR-2 agonists irreversibly diminished voltage-gated Ca(2+) currents (I(Ca)), measured using the patch-clamp method, in rat CG neurons, whereas thrombin had little effect on I(Ca). This PAR-2-induced inhibition was almost completely prevented by omega-CgTx, a potent N-type Ca(2+) channel blocker, suggesting the involvement of N-type Ca(2+) channels in PAR-2-induced inhibition. In addition, PAR-2 agonists inhibited I(Ca-N) in a voltage-independent manner in rat CG neurons. Moreover, PAR-2 agonists reduced action potential (AP) firing frequency as measured using the current-clamp method in rat CG neurons. This inhibition of AP firing induced by PAR-2 agonists was almost completely prevented by omega-CgTx, indicating that PAR-2 activation may regulate the membrane excitability of peripheral sympathetic neurons through modulation of N-type Ca(2+) channels. In conclusion, the present findings demonstrate that the activation of PAR-2 suppresses peripheral sympathetic outflow by modulating N-type Ca(2+) channel activity, which appears to be involved in PAR-2-induced hypotension, in peripheral sympathetic nerve terminals.
Effect of the house dust mite allergen Der p 1 on tryptase release from human mast cells.[Pubmed:27421012]
Genet Mol Res. 2016 Jul 14;15(2). pii: gmr8284.
This study aimed to investigate the effects of the house dust mite allergen Der p 1 on the secretion of tryptase from the human mast cell line HMC-1. Flow cytometry was used to determine the expression levels of protease-activated receptor-2 (PAR2) on the surface of HMC-1 cells. HMC-1 cells were treated with Der p 1, SLIGRL-NH2 (PAR2 agonist), LRGILS-NH2 (control peptide for PAR2), or Der p 1 + FSLLRY (PAR2 antagonist), and the tryptase levels were measured using enzyme-linked immunosorbent assay. The biological functions of PAR2 were determined using the calcium green indicator, and intracellular calcium fluorescence intensity in the different groups (Der p 1, SLIGRL-NH2, LRGILS- NH2, Der p 1 + FSLLRY, tryptase, tryptase + FSLLRY, or cell culture medium) was detected by laser scanning confocal microscopy. The mast cells expressed PAR2 receptor on their surfaces. Der p 1 alone induced a significant release of intracellular calcium and tryptase in HMC-1 cells compared with the SLIGRL- NH2 treatment group and the control group. The combination of Der p 1 and FSLLRY partly inhibited intracellular calcium and tryptase release in HMC-1 cells compared with the Der p 1 treatment group. Moreover, tryptase induced a significant release of intracellular calcium in the HMC-1 cells. Der p 1 induced HMC-1 cell degranulation and the release of tryptase by activating the PAR2 receptor on the cell surfaces. Tryptase activated the PAR2 receptor and induced intracellular calcium release from the HMC-1 cells in a positive feedback loop.
Agonists of protease-activated receptors 1 and 2 stimulate electrolyte secretion from mouse gallbladder.[Pubmed:17431214]
Am J Physiol Gastrointest Liver Physiol. 2007 Jul;293(1):G335-46.
Cholecystitis is one of the most common gastrointestinal diseases. Inflammation induces the activation of proteases that can signal to cells by cleaving protease-activated receptors (PARs) to induce hemostasis, inflammation, pain, and repair. However, the distribution of PARs in the gallbladder is unknown, and their effects on gallbladder function have not been fully investigated. We localized immunoreactive PAR(1) and PAR(2) to the epithelium, muscle, and serosa of mouse gallbladder. mRNA transcripts corresponding to PAR(1) and PAR(2), but not PAR(4), were detected by RT-PCR and sequencing. Addition of thrombin and a PAR(1)-selective activating peptide (TFLLRN-NH(2)) to the serosal surface of mouse gallbladder mounted in an Ussing chamber stimulated an increase in short-circuit current in wild-type but not PAR(1) knockout mice. Similarly, serosally applied trypsin and PAR(2) activating peptide (SLIGRL-NH(2)) increased short-circuit current in wild-type but not PAR(2) knockout mice. Proteases and activating peptides strongly inhibited electrogenic responses to subsequent stimulation with the same agonist, indicating homologous desensitization. Removal of HCO(3)(-) ions from the serosal buffer reduced responses to thrombin and trypsin by >80%. Agonists of PAR(1) and PAR(2) increase intracellular Ca(2+) concentration in isolated and cultured gallbladder epithelial cells. The COX-2 inhibitor meloxicam and an inhibitor of CFTR prevented the stimulatory effect of PAR(1) but not PAR(2). Thus PAR(1) and PAR(2) are expressed in the epithelium of the mouse gallbladder, and serosally applied proteases cause a HCO(3)(-) secretion. The effects of PAR(1) but not PAR(2) depend on generation of prostaglandins and activation of CFTR. These mechanisms may markedly influence fluid and electrolyte secretion of the inflamed gallbladder when multiple proteases are generated.
Stimulation of PAR-2 excites and sensitizes rat cutaneous C-nociceptors to heat.[Pubmed:15486484]
Neuroreport. 2004 Sep 15;15(13):2071-5.
Proteinase-activated receptor 2 (PAR-2) is expressed on many nociceptive neurons. Application of PAR-2 agonists has been shown to induce behavioral signs of hyperalgesia. We investigated effects of the rat PAR-2 agonist SLIGRL-NH2 in the isolated rat skin-saphenous nerve preparation. SLIGRL-NH2 (100 microM) excited 20% of all C-fiber nociceptors tested. In addition, C-fiber nociceptors were sensitized to heat after SLIGRL-NH2 application resulting in an increase in response magnitude and a decrease of heat threshold. The PAR-2-inactive control peptide LRGILS-NH2 had no effect. The mechanical sensitivity of C-fibers was not affected by SLIGRL-NH2. PAR-2-mediated excitation and sensitization of primary nociceptors may contribute to PAR-2-mediated hyperalgesia.
Initial support for the hypothesis that PAR2 is involved in the immune response to Nippostrongylus brasiliensis in mice.[Pubmed:17458579]
Parasitol Res. 2007 Jun;101(1):105-9.
Protease-activated receptor 2 (PAR(2)) is a cell surface receptor that detects trypsin and trypsin-like enzymes. Although the precise pathophysiological roles of PAR(2) are yet to be determined, the receptor has been broadly implicated in inflammation and allergy. However, no studies have investigated the possible roles of PAR(2) in hosts infected by parasitic helminths. Therefore, in this preliminary investigation, we compared the infectivity of the nematode Nippostrongylus brasiliensis in mice lacking the PAR(2) gene (PAR2-/- ) and in their 'background-strain' controls (129SV). PAR2-/- mice displayed elevated fecal egg counts and decreased levels of total serum IgE, after a subcutaneous infection with 900 infective third-stage N. brasiliensis larvae compared with 129SV mice that were not susceptible to infection. In addition, in a separate study in BALB/c mice, two immunological hallmarks of parasite infection, IgE- and IL-10-expressing lymphocytes, were shown to be augmented after the coadministration of the classic antigen ovalbumin with the PAR(2)-activating peptide SLIGRL (single letter amino acid sequence) but not the inactive reverse peptide LRGILS. These findings provide initial support for the proposal that PAR(2) is a recognition receptor for nematode-derived proteases.
Protease-activated receptor-2 (PAR-2)-related peptides induce tear secretion in rats: involvement of PAR-2 and non-PAR-2 mechanisms.[Pubmed:15331653]
J Pharmacol Exp Ther. 2005 Jan;312(1):324-31.
Protease-activated receptor-2 (PAR-2) plays an extensive role in the regulation of digestive exocrine secretion. The present study examined whether PAR-2-related peptides could modulate tear secretion in rats and analyzed the underlying mechanisms. SLIGRL-NH(2), a PAR-2-activating peptide (PAR-2-AP) derived from mouse/rat PAR-2, when administered i.v. in combination with amastatin, an aminopeptidase inhibitor, evoked tear secretion, whereas LRGILS-NH(2), a PAR-2-inactive reversed peptide, had no such effect. In contrast, LSIGRL-NH(2), a partially reversed peptide known to be inactive with PAR-2, caused tear secretion equivalent to the effect of SLIGRL-NH(2). SLIGKV-NH(2), a human-derived PAR-2-AP, also induced significant tear secretion though to a lesser extent, whereas neither VKGILS-NH(2), a reversed peptide, nor LSIGKV-NH(2), a partially reversed peptide, produced any secretion. In desensitization experiments, after the first dose of SLIGRL-NH(2), the second dose of SLIGRL-NH(2) produced no tear secretion, whereas the response to LSIGRL-NH(2) was only partially inhibited by preadministration of SLIGRL-NH(2). Preadministration of LSIGRL-NH(2) abolished the response to subsequently administered LSIGRL-NH(2) but not SLIGRL-NH(2). The tear secretion induced by LSIGRL-NH(2) but not by PAR-2-APs was blocked by atropine or hexamethonium. Mast cell depletion due to repeated doses of compound 48/80 did not alter the effect of SLIGRL-NH(2) or LSIGRL-NH(2). Finally, IGRL-NH(2), a possible core structure of LSIGRL-NH(2), triggered tear secretion in an atropine-reversible manner. Our findings suggest that the PAR-2-APs SLIGRL-NH(2) and SLIGKV-NH(2) cause tear secretion, most likely via PAR-2 and that LSIGRL-NH(2), a PAR-2-inactive peptide, and IGRL-NH(2), its key structure, trigger tear secretion by stimulating parasympathetic nerves via an unidentified target molecule.
Evidence that PAR-1 and PAR-2 mediate prostanoid-dependent contraction in isolated guinea-pig gallbladder.[Pubmed:11030717]
Br J Pharmacol. 2000 Oct;131(4):689-94.
We have investigated the ability of protease-activated receptor-1 (PAR-1), PAR-2, PAR-3 and PAR-4 agonists to induce contractile responses in isolated guinea-pig gallbladder. Thrombin, trypsin, mouse PAR-1 activating (SFLLRN-NH(2)) peptide, and mouse PAR-2 activating (SLIGRL-NH(2)) and human PAR-2 activating (SLIGKV-NH(2)) peptides produced a concentration-dependent contractile response. Mouse PAR-4 activating (GYPGKF-NH(2)) peptide, the mouse PAR-1 reverse (NRLLFS-NH(2)) peptide, the mouse PAR-2 reverse (LRGILS-NH(2)) and human PAR-2 reverse (VKGILS-NH(2)) peptides caused negligible contractile responses at the highest concentrations tested. An additive effect was observed following the contractile response induced by either trypsin or thrombin, with the addition of a different PAR agonist (SFLLRN-NH(2) and SLIGRL-NH(2), respectively). Desensitization to PAR-2 activating peptide attenuated the response to trypsin but failed to attenuate the response to PAR-1 agonists, and conversely desensitization to PAR-1 attenuated the response to thrombin but failed to alter contractile responses to PAR-2 agonists. The contractile responses produced by thrombin, trypsin, SFLLRN-NH(2) and SLIGRL-NH(2) were markedly reduced in the presence of the cyclo-oxygenase inhibitor, indomethacin, whilst the small contractile response produced by NRLLFS-NH(2) and LRGILS-NH(2) were insensitive to indomethacin. The contractile responses to thrombin, trypsin, SFLLRN-NH(2) and SLIGRL-NH(2) were unaffected by the presence of: the non-selective muscarinic antagonist, atropine; the nitric oxide synthase inhibitor, L-NAME; the sodium channel blocker, tetrodotoxin; the combination of selective tachykinin NK(1) and NK(2) receptor antagonists, (S)-1-[2-[3-(3,4-dichlorphenyl)-1 (3-isopropoxyphenylacetyl) piperidin-3-yl] ethyl]-4-phenyl-1 azaniabicyclo [2.2.2] octane chloride (SR140333) and (S)-N-methyl-N-[4-acetylamino-4-phenylpiperidino-2-(3, 4-dichlorophenyl)-butyl] benzamide (SR48968), respectively. The results indicate that PAR-1 and PAR-2 activation causes contractile responses in the guinea-pig gallbladder, an effect that is mediated principally by prostanoid release, and is independent of neural mechanisms.


