MaclurinCAS# 519-34-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 519-34-6 | SDF | Download SDF |
| PubChem ID | 68213 | Appearance | Yellow powder |
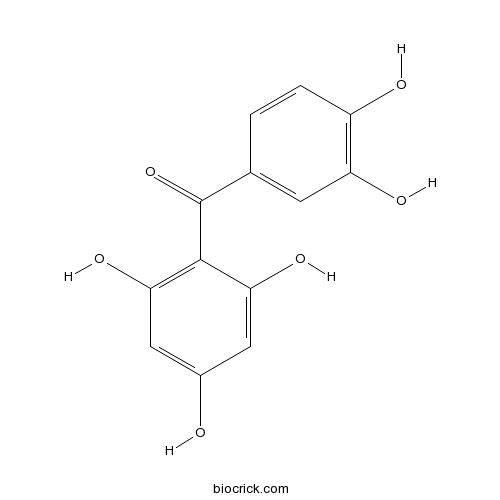
| Formula | C13H10O6 | M.Wt | 262.2 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3,4-dihydroxyphenyl)-(2,4,6-trihydroxyphenyl)methanone | ||
| SMILES | C1=CC(=C(C=C1C(=O)C2=C(C=C(C=C2O)O)O)O)O | ||
| Standard InChIKey | XNWPXDGRBWJIES-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H10O6/c14-7-4-10(17)12(11(18)5-7)13(19)6-1-2-8(15)9(16)3-6/h1-5,14-18H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Maclurin can effectively protect against mesenchymal stem cells (MSCs) oxidative damage induced by hydroxyl radical (OH) at 62.1-310.5 uM, the protective and antioxidant effects of maclurin can be primarily attributed to ortho-dihydroxyl groups, and ultimately to the relative stability of the ortho-benzoquinone form. 2. Maclurin has anti-metastatic effect, the mechanism is by anti-oxidative activity and inhibition of Src/FAK-ERK-β-catenin signaling pathway. |
| Targets | ROS | Src | FAK | ERK | Wnt/β-catenin | GSK-3 | MMP(e.g.TIMP) |

Maclurin Dilution Calculator

Maclurin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8139 mL | 19.0694 mL | 38.1388 mL | 76.2777 mL | 95.3471 mL |
| 5 mM | 0.7628 mL | 3.8139 mL | 7.6278 mL | 15.2555 mL | 19.0694 mL |
| 10 mM | 0.3814 mL | 1.9069 mL | 3.8139 mL | 7.6278 mL | 9.5347 mL |
| 50 mM | 0.0763 mL | 0.3814 mL | 0.7628 mL | 1.5256 mL | 1.9069 mL |
| 100 mM | 0.0381 mL | 0.1907 mL | 0.3814 mL | 0.7628 mL | 0.9535 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ellipticine
Catalog No.:BCC7665
CAS No.:519-23-3
- Benzoylecgonine
Catalog No.:BCN1909
CAS No.:519-09-5
- Matrine
Catalog No.:BCN5650
CAS No.:519-02-8
- Pizotifen Malate
Catalog No.:BCC4825
CAS No.:5189-11-7
- Axillarin
Catalog No.:BCN8102
CAS No.:5188-73-8
- 7-O-Methyleriodictyol
Catalog No.:BCN5648
CAS No.:51857-11-5
- Licoricone
Catalog No.:BCN6818
CAS No.:51847-92-8
- Allamandicin
Catalog No.:BCN4625
CAS No.:51838-83-6
- 3-MATIDA
Catalog No.:BCC7281
CAS No.:518357-51-2
- Angiotensin (1-7)
Catalog No.:BCC1029
CAS No.:51833-78-4
- Angiotensin 1/2 + A (2 - 8)
Catalog No.:BCC1037
CAS No.:51833-76-2
- UMI-77
Catalog No.:BCC5567
CAS No.:518303-20-3
- Sulochrin
Catalog No.:BCN6959
CAS No.:519-57-3
- 4-(N-Methyl)-aminoantipyrine
Catalog No.:BCC8652
CAS No.:519-98-2
- Dehydrohautriwaic acid
Catalog No.:BCN7586
CAS No.:51905-84-1
- Tasisulam
Catalog No.:BCC4407
CAS No.:519055-62-0
- CJ 033466
Catalog No.:BCC7562
CAS No.:519148-48-2
- TNP
Catalog No.:BCC7822
CAS No.:519178-28-0
- 5-Aminoindole
Catalog No.:BCC8735
CAS No.:5192-03-0
- Schaftoside
Catalog No.:BCN2343
CAS No.:51938-32-0
- Dehydroespeletone
Catalog No.:BCN5652
CAS No.:51995-99-4
- Spironolactone
Catalog No.:BCC4366
CAS No.:52-01-7
- Prednisolone Acetate
Catalog No.:BCC4831
CAS No.:52-21-1
- Thio-TEPA
Catalog No.:BCC5354
CAS No.:52-24-4
Maclurin suppresses migration and invasion of human non-small-cell lung cancer cells via anti-oxidative activity and inhibition of the Src/FAK-ERK-beta-catenin pathway.[Pubmed:25630491]
Mol Cell Biochem. 2015 Apr;402(1-2):243-52.
Recent reports indicated that ROS is closely related with cancer metastasis. ROS targets major signaling molecules which are known to be involved in migration and invasion of cancer cells. Here we report that Maclurin, a major phenolic component of ethanol extracted mulberry twigs, exerts anti-metastatic effect in A549 human non-small-cell lung cancer cells. Maclurin suppresses intracellular ROS level in A549 human non-small-cell lung cancer cells. Also, Maclurin down-regulates Src and ERK, which are well known to be regulated with redox state. Suppressed Src/FAK and ERK signalings activate GSK3-beta, thus inhibiting nuclear accumulation of beta-catenin. As a result, transcriptional expressions of two major gelatinases (MMP-2 and MMP-9) were significantly down-regulated. Consequently, migration and invasion of A549 human non-small-cell lung cancer cells were attenuated. Anti-metastatic effect of Maclurin on A549 human non-small-cell lung cancer cells were diminished by the treatment of hydrogen peroxide, thus further implicating that the effect of Maclurin may be strongly related with its anti-oxidative activity. Thus, our data indicate that the anti-metastatic effect of Maclurin is exerted by anti-oxidative activity and inhibition of Src/FAK-ERK-beta-catenin signaling pathway.
Maclurin protects against hydroxyl radical-induced damages to mesenchymal stem cells: antioxidant evaluation and mechanistic insight.[Pubmed:24973644]
Chem Biol Interact. 2014 Aug 5;219:221-8.
Maclurin, an exceptional member of phytophenol family, was found to effectively protect against mesenchymal stem cells (MSCs) oxidative damage induced by hydroxyl radical (OH) at 62.1-310.5 muM. Antioxidant assays indicated that Maclurin could efficiently protect DNA from OH-induced damage at 114.6-382.2 muM, and scavenge OH, DPPH (1,1-diphenyl-2-picrylhydrazyl radical), ABTS(+) (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid radical), and bind Cu(2+) (IC50 values were respectively 122.87 +/- 10.14, 10.15 +/- 0.85, 0.97 +/- 0.07, and 133.95 +/- 11.92 muM). HPLC-DAD and HPLC-ESI-MS/MS analyses of the end-product of Maclurin reaction with DPPH clearly suggested that Maclurin (m/z = 261.12 [M-H](-)) donated two hydrogen atoms to DPPH (m/z = 394.06 [M](+)) to form ortho-benzoquinone moiety (lambdamax = 364 nm; m/z = 259.06 [M-H](-), loss of m/z = 28) and DPPH2 molecule (m/z = 395.03, 396.01), via hydrogen atom transfer (HAT) or sequential electron (e) proton transfer (SEPT), not radical adduct formation (RAF) mechanisms. Therefore, we concluded that: (i) Maclurin can effectively protect against OH-induced damages to DNA and MSCs, thereby it may have a therapeutic potential in prevention of many diseases or MSCs transplantation; (ii) a possible mechanism for Maclurin to protect against oxidative damages is OH radical-scavenging; (iii) Maclurin scavenges OH possibly through metal-chelating, and direct radical-scavenging which is mainly via HAT or SEPT mechanisms; and (iv) the protective and antioxidant effects of Maclurin can be primarily attributed to ortho-dihydroxyl groups, and ultimately to the relative stability of the ortho-benzoquinone form.


