Angiotensin (1-7)Vasoconstriction peptide hormone CAS# 51833-78-4 |

- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- Bay 65-1942 free base
Catalog No.:BCC1408
CAS No.:600734-02-9
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 51833-78-4 | SDF | Download SDF |
| PubChem ID | 123805 | Appearance | Powder |
| Formula | C41H62N12O11 | M.Wt | 899 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Angiotensin-(1-7); Ang-(1-7) | ||
| Solubility | H2O : ≥ 30.2 mg/mL (33.59 mM) *"≥" means soluble, but saturation unknown. | ||
| Sequence | DRVYIHP | ||
| Chemical Name | (2S)-1-[(2S)-2-[[(2S,3S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-carboxypropanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-methylpentanoyl]amino]-3-(1H-imidazol-5-yl)propanoyl]pyrrolidine-2-carboxylic acid | ||
| SMILES | CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc2[nH]cnc2)C(=O)N3CCC[C@H]3C(O)=O | ||
| Standard InChIKey | PVHLMTREZMEJCG-GDTLVBQBSA-N | ||
| Standard InChI | InChI=1S/C41H62N12O11/c1-5-22(4)33(38(61)50-29(17-24-19-45-20-47-24)39(62)53-15-7-9-30(53)40(63)64)52-36(59)28(16-23-10-12-25(54)13-11-23)49-37(60)32(21(2)3)51-35(58)27(8-6-14-46-41(43)44)48-34(57)26(42)18-31(55)56/h10-13,19-22,26-30,32-33,54H,5-9,14-18,42H2,1-4H3,(H,45,47)(H,48,57)(H,49,60)(H,50,61)(H,51,58)(H,52,59)(H,55,56)(H,63,64)(H4,43,44,46)/t22-,26-,27-,28-,29-,30-,32-,33-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous peptide fragment. Induces vasorelaxation through release of NO and prostaglandins, perhaps through activation of a non-AT1, non-AT2 receptor, Mas. Counteracts the vasoconstrictive and proliferative effects of angiotensin II and stimulates vasopressin (anti-diuretic hormone) release in vivo. |

Angiotensin (1-7) Dilution Calculator

Angiotensin (1-7) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ang-(1-7) (H - Asp - Arg - Val - Tyr - Ile - His - Pro - OH) is an endogenous peptide fragment that can be produced from Ang I or Ang II via endo- or carboxy-peptidases respectively[1].
As described for Ang II, Ang-(1-7) also has a broad range of effects in different organs and tissues and goes beyond its initially described cardiovascular and renal actions. Those effects are mediated by Mas and can counter-regulate most of the deleterious effects of Ang II. The interaction Ang-(1-7)/Mas regulates different signaling pathways, such as PI3K (phosphoinositide 3-kinase)/AKT and ERK (extracellular signal-regulated kinase) pathways and involves downstream effectors such as NO, FOXO1 (forkhead box O1) and COX-2 (cyclo-oxygenase-2). Through these mechanisms, Ang-(1-7) is able to improve pathological conditions including fibrosis and inflammation in organs such as lungs, liver and kidney. [2]
In addition, this heptapeptide has positive effects on metabolism, increasing the glucose uptake and lipolysis while decreasing insulin resistance and dyslipidemia. Ang-(1-7) is also able to improve cerebroprotection against ischemic stroke, besides its effects on learning and memory. The reproductive system can also be affected by Ang-(1-7) treatment, with enhanced ovulation, spermatogenesis and sexual steroids synthesis. Finally, Ang-(1-7) is considered a potential anti-cancer treatment since it is able to inhibit cell proliferation and angiogenesis.[2]
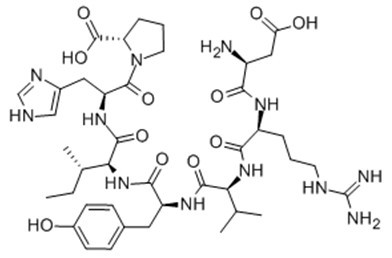
Fig. 1: Formula of Angiotensin (1-7)
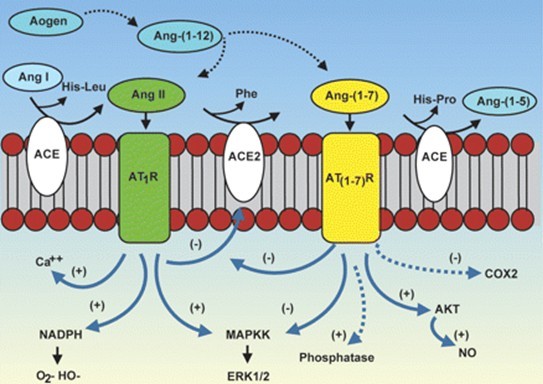
Fig. 2: Cascade of the processing of angiotensin peptides and their interaction with AT1 and AT1-7 receptor systems.
Ref:
1. Santos et al (2000) Angiotensin-(1-7): an update. Regul.Pept. 91 45.
2. Danielle G. P., Thiago V., Robson A. S. Angiotensin-(1-7): beyond the cardio-renal actions. Clinical Science (2013) 124, (443–456)
- Angiotensin 1/2 + A (2 - 8)
Catalog No.:BCC1037
CAS No.:51833-76-2
- UMI-77
Catalog No.:BCC5567
CAS No.:518303-20-3
- 2',4'-Dihydroxychalcone
Catalog No.:BCN5647
CAS No.:1776-30-3
- KX1-004
Catalog No.:BCC5440
CAS No.:518058-84-9
- Raltegravir (MK-0518)
Catalog No.:BCC2137
CAS No.:518048-05-0
- Dihydrooxoepistephamiersine
Catalog No.:BCN5646
CAS No.:51804-69-4
- Oxoepistephamiersine
Catalog No.:BCN5645
CAS No.:51804-68-3
- Nimesulide
Catalog No.:BCC4435
CAS No.:51803-78-2
- 3,3'-Di-O-methylellagic acid 4'-glucoside
Catalog No.:BCN1431
CAS No.:51803-68-0
- Isomaculosidine
Catalog No.:BCN7069
CAS No.:518-96-7
- Cycleanine
Catalog No.:BCN8445
CAS No.:518-94-5
- Xanthopurpurin
Catalog No.:BCN6723
CAS No.:518-83-2
- 3-MATIDA
Catalog No.:BCC7281
CAS No.:518357-51-2
- Allamandicin
Catalog No.:BCN4625
CAS No.:51838-83-6
- Licoricone
Catalog No.:BCN6818
CAS No.:51847-92-8
- 7-O-Methyleriodictyol
Catalog No.:BCN5648
CAS No.:51857-11-5
- Axillarin
Catalog No.:BCN8102
CAS No.:5188-73-8
- Pizotifen Malate
Catalog No.:BCC4825
CAS No.:5189-11-7
- Matrine
Catalog No.:BCN5650
CAS No.:519-02-8
- Benzoylecgonine
Catalog No.:BCN1909
CAS No.:519-09-5
- Ellipticine
Catalog No.:BCC7665
CAS No.:519-23-3
- Maclurin
Catalog No.:BCN5651
CAS No.:519-34-6
- Sulochrin
Catalog No.:BCN6959
CAS No.:519-57-3
- 4-(N-Methyl)-aminoantipyrine
Catalog No.:BCC8652
CAS No.:519-98-2
Angiotensin-(1-7) Downregulates Diabetes-Induced cGMP Phosphodiesterase Activation in Rat Corpus Cavernosum.[Pubmed:28299329]
Biomed Res Int. 2017;2017:5084961.
Molecular mechanisms of the beneficial effects of angiotensin-(1-7), Ang-(1-7), in diabetes-related complications, including erectile dysfunction, remain unclear. We examined the effect of diabetes and/or Ang-(1-7) treatment on vascular reactivity and cyclic guanosine monophosphate (cGMP) phosphodiesterase (PDE) in corpus cavernosum. Male Wistar rats were grouped as (1) control, (2) diabetic (streptozotocin, STZ, treated), (3) control + Ang-(1-7), and (4) diabetic + Ang-(1-7). Following 3 weeks of Ang-(1-7) treatment subsequent to induction of diabetes, rats were sacrificed. Penile cavernosal tissue was isolated to measure vascular reactivity, PDE gene expression and activity, and levels of p38MAP kinase, nitrites, and cGMP. Carbachol-induced vasorelaxant response after preincubation of corpus cavernosum with PE was significantly attenuated in diabetic rats, and Ang-(1-7) markedly corrected the diabetes-induced impairment. Gene expression and activity of PDE and p38MAP kinase were significantly increased in cavernosal tissue of diabetic rats, and Ang-(1-7) markedly attenuated STZ-induced effects. Ang-(1-7) significantly increased the levels of nitrite and cGMP in cavernosal tissue of control and diabetic rats. Cavernosal tissue of diabetic rats had significantly reduced cGMP levels and Ang-(1-7) markedly prevented the STZ-induced cGMP depletion. This study demonstrates that attenuation of diabetes-induced PDE activity might be one of the key mechanisms in the beneficial effects of Ang-(1-7).
[Effects and related mechanism of angiotensin-(1-7) on Toll-like receptor 4-mediated oxidative stress in human umbilical vein endothelial cells].[Pubmed:28316179]
Zhonghua Xin Xue Guan Bing Za Zhi. 2017 Mar 24;45(3):223-229.
Objective: To explore the role and related mechanisms of angiotensin-(1-7)(Ang-(1-7)) on Toll-like receptor 4 (TLR4) mediated oxidized low-density lipoprotein(ox-LDL)-induced oxidative stress in human umbilical vein endothelial cells (HUVECs). Methods: HUVECs were cultured in vitro and divided into six groups: the control group (normal medium), the ox-LDL group(treated with 75 mg/L ox-LDL), the ox-LDL+ Ang-(1-7) group (1 mumol/L Ang-(1-7) pretreated for 30 minutes, then intervened with 75 mg/L ox-LDL), the ox-LDL+ Ang-(1-7)+ A-779 group(1 mumol/L A-779 (Mas receptor) pretreated for 30 minutes, 1 mumol/L Ang-(1-7) pretreated for 30 minutes, then intervened with 75 mg/L ox-LDL), the ox-LDL+ A-779 group (1 mumol/L A-779 pretreated for 30 minutes, then intervened with 75 mg/L ox-LDL), the ox-LDL+ HTA125 group (10 mug/L HTA125 (TLR4-blocking antibody) pretreated for 30 minutes, then intervened with 75 mg/L ox-LDL ). The corresponding index was detected after 24 hours after intervention. Apoptosis of cells were detected by Annexin V-FITC/PI double staining flow cytometry and transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL). The generation of reactive oxygen species (ROS), products in oxidative stress, were detected by DCFH-DA staining. The mRNA and protein expression levels of NADPH oxidase 4(NOX4) and TLR4 were detected by real-time reverse transcription-polymerase chain reaction (RT-PCR) and Western blotting analysis respectively. Results: (1) The results of Annexin V-FITC/PI double staining flow cytometry showed that the proportion of apoptotic cells was higher in ox-LDL group than in control group ((21.18+/-1.40)% vs. (1.59+/-0.26)%, P<0.01), lower in ox-LDL+ Ang-(1-7) group((7.42+/-1.07)%) and ox-LDL+ HTA125 group((9.19+/-1.01)%) than in ox-LDL group (both P<0.01), higher in ox-LDL+ Ang-(1-7)+ A-779 group ((19.91+/-1.30)%) and ox-LDL+ A-779 group((20.47+/-0.95)%) than in ox-LDL+ Ang-(1-7) group (both P<0.01). (2) The TUNEL results showed that the proportion of apoptotic cells was higher in ox-LDL group than in control group((10.83+/-0.77)% vs. (2.83+/-0.82)%, P<0.01), lower in ox-LDL+ Ang-(1-7) group ((3.66+/-0.54)%)and ox-LDL+ HTA125 group((4.97+/-0.60)%) than in ox-LDL group(both P<0.01), higher in ox-LDL+ Ang-(1-7)+ A-779 group((10.69+/-0.62)%) and ox-LDL+ A-779 group((11.43+/-0.42)%) than in ox-LDL+ Ang-(1-7) group (both P<0.01). (3) ROS level was higher in ox-LDL group than in control group(0.093+/-0.014 vs. 0.053+/-0.011, P<0.01), lower in ox-LDL+ Ang-(1-7) group (0.063+/-0.011, P<0.01)and ox-LDL+ HTA125 group(0.070+/-0.010, P<0.05)than in ox-LDL group, higher in ox-LDL+ Ang-(1-7)+ A-779 group(0.088+/-0.003) and ox-LDL+ A-779 group(0.095+/-0.005) than in ox-LDL+ Ang-(1-7) group (both P<0.01). (4) The mRNA expression level of NOX4 was higher in ox-LDL group than in control group(11.74+/-0.65 vs. 1.00+/-0.00, P<0.01), lower in ox-LDL+ Ang-(1-7) group (2.85+/-0.75)and ox-LDL+ HTA125 group(5.57+/-0.52) than in ox-LDL group(both P<0.01), higher in ox-LDL+ Ang-(1-7)+ A-779 group(10.51+/-0.54) and ox-LDL+ A-779 group (11.04+/-1.01) than in ox-LDL+ Ang-(1-7) group (both P<0.01), higher in ox-LDL group than in control group(27.60+/-1.86 vs. 1.00+/-0.00, P<0.01), lower in ox-LDL+ Ang-(1-7) group (8.00+/-1.03)and ox-LDL+ HTA125 group(14.83+/-0.97)than in ox-LDL group(both P<0.01), higher in ox-LDL+ Ang-(1-7)+ A-779 group(24.81+/-2.19) and ox-LDL+ A-779 group (26.64+/-0.65)than in ox-LDL+ Ang-(1-7) group (both P<0.01). (5)The protein expression level of NOX4 was higher in ox-LDL group than in control group (0.61+/-0.09 vs. 0.23+/-0.02, P<0.01), lower in ox-LDL+ Ang-(1-7) group(0.27+/-0.03) and ox-LDL+ HTA125 group(0.22+/-0.02) than in ox-LDL group(both P<0.01), higher in ox-LDL+ Ang-(1-7)+ A-779 group (0.58+/-0.06)and ox-LDL+ A-779 group(0.61+/-0.03) than in ox-LDL+ Ang-(1-7) group (both P<0.01). The protein expression level of TLR4 was higher in ox-LDL group than in control group(0.18+/-0.02 vs. 0.08+/-0.01, P<0.01), lower in ox-LDL+ Ang-(1-7) group(0.07+/-0.01) and ox-LDL+ HTA125 group(0.09+/-0.01) than in ox-LDL group(both P<0.01), higher in ox-LDL+ Ang-(1-7)+ A-779 group(0.18+/-0.02) and ox-LDL+ A-779 group(0.20+/-0.02) than in ox-LDL+ Ang-(1-7) group (both P<0.01). Conclusion: TLR4 mediated the ox-LDL induced injury in HUVECs, and Ang-(1-7) could attenuate ox-LDL induced injury in HUVECs by modulating the specific Mas receptors.
Angiotensin (1-7) facilitates cardioprotection of ischemic preconditioning on ischemia-reperfusion-challenged rat heart.[Pubmed:28293875]
Mol Cell Biochem. 2017 Jun;430(1-2):99-113.
Ischemic preconditioning (IPC) is one of the most promising strategies used to protect the myocardium from ischemia-reperfusion injury. Ang (1-7) exhibits cardioprotective activity; however, its therapeutic potential on IPC-induced cardioprotection has not been reported in ischemia-reperfusion injury till date. Therefore, the first set of experiment was designed to evaluate the direct effect of Ang (1-7), in perfusion medium, on cardioprotective activity of IPC in rat heart challenged to ischemia-reperfusion injury. In addition, the acute and chronic effects of pretreated Ang (1-7) were investigated on cardioprotection of IPC in ischemia-reperfusion-challenged hearts in subsequent sets of experiments. The results showed that Ang (1-7) potentiated the IPC-induced increase in coronary flow and heart rate, decrease in lactate dehydrogenase and creatine kinase activity, ventricular fibrillation, and infarct size in ischemia-reperfusion-challenged animals in direct and chronic sets of experiments. Further, Ang (1-7) enhanced the IPC-induced attenuation in mitochondrial dysfunction, oxidative stress, and apoptosis in ischemia-reperfusion-challenged hearts in both sets of experiments. A-779, Mas receptor antagonist, abrogated potentiation effects of Ang (1-7) on IPC-induced cardioprotection in ischemia-reperfusion-challenged rats in the above set of experiments. These observations indicate that Ang (1-7)/Mas receptor activation could be a potential adjuvant to IPC during ischemia-reperfusion-induced cardiac injury.
Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas.[Pubmed:12829792]
Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8258-63.
The renin-angiotensin system plays a critical role in blood pressure control and body fluid and electrolyte homeostasis. Besides angiotensin (Ang) II, other Ang peptides, such as Ang III [Ang-(2-8)], Ang IV [Ang-(3-8)], and Ang-(1-7) may also have important biological activities. Ang-(1-7) has become an angiotensin of interest in the past few years, because its cardiovascular and baroreflex actions counteract those of Ang II. Unique angiotensin-binding sites specific for this heptapeptide and studies with a selective Ang-(1-7) antagonist indicated the existence of a distinct Ang-(1-7) receptor. We demonstrate that genetic deletion of the G protein-coupled receptor encoded by the Mas protooncogene abolishes the binding of Ang-(1-7) to mouse kidneys. Accordingly, Mas-deficient mice completely lack the antidiuretic action of Ang-(1-7) after an acute water load. Ang-(1-7) binds to Mas-transfected cells and elicits arachidonic acid release. Furthermore, Mas-deficient aortas lose their Ang-(1-7)-induced relaxation response. Collectively, these findings identify Mas as a functional receptor for Ang-(1-7) and provide a clear molecular basis for the physiological actions of this biologically active peptide.
Angiotensin-(1-7) is involved in the endothelium-dependent modulation of phenylephrine-induced contraction in the aorta of mRen-2 transgenic rats.[Pubmed:11934815]
Br J Pharmacol. 2002 Apr;135(7):1743-8.
1. The contribution of the local vascular production of angiotensin-(1-7) [Ang-(1-7)] to the control of alpha-adrenergic-induced contractions in the aorta of Sprague-Dawley (SD) and TGR(mRen-2)27 [mRen-2] rats was studied. 2. In mRen-2 rats, contractile responses to phenylephrine were diminished as compared to control SD rats in endothelium containing but not in endothelium-denuded vessels. L-NAME increased contractile responses to phenylephrine in mRen-2 rats and, after nitric oxide synthase blockade, responses to phenylephrine became comparable in both strains. 3. Inhibition of angiotensin-converting enzyme (ACE) by captopril potentiated contractile responses in mRen-2 rats and diminished contractile responses in SD rats, both effects being dependent on the presence of a functional endothelium. The effect of captopril in mRen-2 rats was abolished in vessels pre-incubated with Ang-(1-7). 4. Blockade of Ang-(1-7) and bradykinin (BK) receptors by A-779 and HOE 140 respectively, increased phenylephrine-induced contraction in mRen-2, but not in SD rats. This effect was seen only in endothelium-containing vessels. 5. Angiotensin II AT(1) and AT(2) receptor blockade by CV 11974 and PD 123319 did not affect the contractile responses to phenylephrine in aortas of transgenic animals but diminished the response in SD rats. This effect was only seen in the presence of a functional endothelium. 6. It is concluded that the decreased contractile responses to phenylephrine in aortas of mRen-2 rats was dependent on an intact endothelium, the local release and action of Ang-(1-7) and bradykinin.
Angiotensin-(1-7): an update.[Pubmed:10967201]
Regul Pept. 2000 Jul 28;91(1-3):45-62.
The renin-angiotensin system is a major physiological regulator of arterial pressure and hydro-electrolyte balance. Evidence has now been accumulated that in addition to angiotensin (Ang) II other Ang peptides [Ang III, Ang IV and Ang-(1-7)], formed in the limited proteolysis processing of angiotensinogen, are importantly involved in mediating several actions of the RAS. In this article we will review our knowledge of the biological actions of Ang-(1-7) with focus on the puzzling aspects of the mediation of its effects and the interaction Ang-(1-7)-kinins. In addition, we will attempt to summarize the evidence that Ang-(1-7) takes an important part of the mechanisms aimed to counteract the vasoconstrictor and proliferative effects of Ang II.


