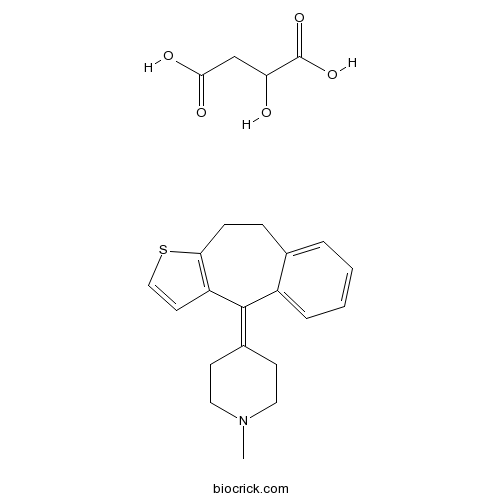
Pizotifen MalateCAS# 5189-11-7 |
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- Mianserin HCl
Catalog No.:BCC1114
CAS No.:21535-47-7
- Loratadine
Catalog No.:BCC1262
CAS No.:79794-75-5
- Bavisant
Catalog No.:BCC1402
CAS No.:929622-08-2
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5189-11-7 | SDF | Download SDF |
| PubChem ID | 168993 | Appearance | Powder |
| Formula | C23H27NO5S | M.Wt | 429.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
| Chemical Name | 4-(4,5-dihydrobenzo[1,2]cyclohepta[3,4-b]thiophen-10-ylidene)-1-methylpiperidine;2-hydroxybutanedioic acid | ||
| SMILES | CN1CCC(=C2C3=C(CCC4=CC=CC=C42)SC=C3)CC1.C(C(C(=O)O)O)C(=O)O | ||
| Standard InChIKey | IWAWCPZVTXCFKD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H21NS.C4H6O5/c1-20-11-8-15(9-12-20)19-16-5-3-2-4-14(16)6-7-18-17(19)10-13-21-18;5-2(4(8)9)1-3(6)7/h2-5,10,13H,6-9,11-12H2,1H3;2,5H,1H2,(H,6,7)(H,8,9) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Pizotifen Malate Dilution Calculator

Pizotifen Malate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3281 mL | 11.6406 mL | 23.2813 mL | 46.5625 mL | 58.2032 mL |
| 5 mM | 0.4656 mL | 2.3281 mL | 4.6563 mL | 9.3125 mL | 11.6406 mL |
| 10 mM | 0.2328 mL | 1.1641 mL | 2.3281 mL | 4.6563 mL | 5.8203 mL |
| 50 mM | 0.0466 mL | 0.2328 mL | 0.4656 mL | 0.9313 mL | 1.1641 mL |
| 100 mM | 0.0233 mL | 0.1164 mL | 0.2328 mL | 0.4656 mL | 0.582 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pizotifen malate is a highly selective 5-HT receptor blocking agent, which is a benzocycloheptane based drug.
- Axillarin
Catalog No.:BCN8102
CAS No.:5188-73-8
- 7-O-Methyleriodictyol
Catalog No.:BCN5648
CAS No.:51857-11-5
- Licoricone
Catalog No.:BCN6818
CAS No.:51847-92-8
- Allamandicin
Catalog No.:BCN4625
CAS No.:51838-83-6
- 3-MATIDA
Catalog No.:BCC7281
CAS No.:518357-51-2
- Angiotensin (1-7)
Catalog No.:BCC1029
CAS No.:51833-78-4
- Angiotensin 1/2 + A (2 - 8)
Catalog No.:BCC1037
CAS No.:51833-76-2
- UMI-77
Catalog No.:BCC5567
CAS No.:518303-20-3
- 2',4'-Dihydroxychalcone
Catalog No.:BCN5647
CAS No.:1776-30-3
- KX1-004
Catalog No.:BCC5440
CAS No.:518058-84-9
- Raltegravir (MK-0518)
Catalog No.:BCC2137
CAS No.:518048-05-0
- Dihydrooxoepistephamiersine
Catalog No.:BCN5646
CAS No.:51804-69-4
- Matrine
Catalog No.:BCN5650
CAS No.:519-02-8
- Benzoylecgonine
Catalog No.:BCN1909
CAS No.:519-09-5
- Ellipticine
Catalog No.:BCC7665
CAS No.:519-23-3
- Maclurin
Catalog No.:BCN5651
CAS No.:519-34-6
- Sulochrin
Catalog No.:BCN6959
CAS No.:519-57-3
- 4-(N-Methyl)-aminoantipyrine
Catalog No.:BCC8652
CAS No.:519-98-2
- Dehydrohautriwaic acid
Catalog No.:BCN7586
CAS No.:51905-84-1
- Tasisulam
Catalog No.:BCC4407
CAS No.:519055-62-0
- CJ 033466
Catalog No.:BCC7562
CAS No.:519148-48-2
- TNP
Catalog No.:BCC7822
CAS No.:519178-28-0
- 5-Aminoindole
Catalog No.:BCC8735
CAS No.:5192-03-0
- Schaftoside
Catalog No.:BCN2343
CAS No.:51938-32-0
Development of antimigraine transdermal delivery systems of pizotifen malate.[Pubmed:26196273]
Int J Pharm. 2015 Aug 15;492(1-2):223-32.
The aim of this study was to develop and evaluate a transdermal delivery system of Pizotifen Malate. Pizotifen is frequently used in the preventive treatment of migraine, but is also indicated in eating disorders. In the course of the project, the effects of chemical enhancers such as ethanol, 1,8-cineole, limonene, azone and different fatty acids (decanoic, decenoic, dodecanoic, linoleic and oleic acids) were determined, first using a pizotifen solution. Steady state flux, diffusion and partition parameters were estimated by fitting the Scheuplein equation to the data obtained. Among the chemical enhancers studied, decenoic acid showed the highest enhancement activity, which seemed to be due to the length of its alkyl chain and unsaturation at the 9th carbon. The influence of iontophoresis and the involvement of electrotransport in said process was determined. The absorption profile obtained with iontophoresis was similar to that obtained with fatty acids and terpenes, though skin deposition of the drug was lower with the former. Transdermal delivery systems (TDS) of pizotifen were manufactured by including chemical enhancers, decenoic acid or oleic acid, and were subsequently characterized. When the results obtained with solutions were compared with those obtained with the TDS, a positive enhancement effect was observed with the latter with respect to the partitioning and diffusion of the drug across the skin. Our findings endorse the suitability of our TDS for delivering therapeutic amounts of Pizotifen Malate.
Solubility of solid dispersions of pizotifen malate and povidone.[Pubmed:11548858]
Drug Dev Ind Pharm. 2001 Jul;27(6):517-22.
We analyzed the physicochemical characteristics of solid dispersions of Pizotifen Malate and povidone (Kollidon 12) at different proportions; we used X-ray diffraction, infrared spectrometry and differential scanning calorimetry (DSC) and tested the solubility of the solid dispersions in equilibrium. The results were compared with findings for physical mixtures with the same proportions. A solid dispersion with a drug proportion of 16%-17% formed a eutectic mixture. Solubility of Pizotifen Malate increased with the proportion of drug in the solid dispersion up to a drug:polymer ratio of 40:60. The hydrotropic effect of the polymer also favored solubility: In physical mixtures, this effect was greatest at a drug:polymer ratio of 10:90; solubility at this proportion was equal to that of the solid dispersion at the same proportion.
Validation and application of reversed phase high-performance liquid chromatographic method for quantification of pizotifen malate in pharmaceutical solid dosage formulations.[Pubmed:20884459]
Pak J Pharm Sci. 2010 Oct;23(4):435-41.
The aim of this study was to develop and validate an isocratic reversed phase high-performance liquid chromatographic method for quantification of Pizotifen Malate in pharmaceutical solid dosage formulations. Good chromatographic separation of Pizotifen Malate was achieved by using an analytical column, C(18) ODS column. The system was operated at 40 degrees C oven temperature using a mobile phase consisting of acetonitrile and acetate buffer pH 7.0 (60:40) at a flow rate of 2 ml/min. The method showed high sensitivity with good linearity (r(2)= 0.99997) over the tested concentration range of 0.0020-0.0300 mg/ml for Pizotifen Malate. Detection was carried out at 231 nm and retention time was 2.838 min. Placebo and blank studies were performed and no peak was observed at the retention time of Pizotifen Malate. The intermediate precision and accuracy results (mean +/- RSD, n=3) were (99.11+/-0.21) % and (99.19+/-0.55) % respectively with tailing factor (1.26+/-0.19). The proposed method was validated in terms of selectivity, linearity, accuracy, precision, range, detection and quantitation limit, system suitability and solution stability.This method can be successfully employed for simultaneous quantitative analysis of Pizotifen Malate in pharmaceutical solid dosage formulations.


