TNPInhibitor of IP6K; also inhibits IP3K CAS# 519178-28-0 |
- Valproic acid sodium salt (Sodium valproate)
Catalog No.:BCC2156
CAS No.:1069-66-5
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- Resminostat (RAS2410)
Catalog No.:BCC2165
CAS No.:864814-88-0
- PCI-34051
Catalog No.:BCC2148
CAS No.:950762-95-5
- Droxinostat
Catalog No.:BCC2157
CAS No.:99873-43-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 519178-28-0 | SDF | Download SDF |
| PubChem ID | 16760513 | Appearance | Powder |
| Formula | C20H16F3N7O2 | M.Wt | 443.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 125 mg/mL (281.93 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
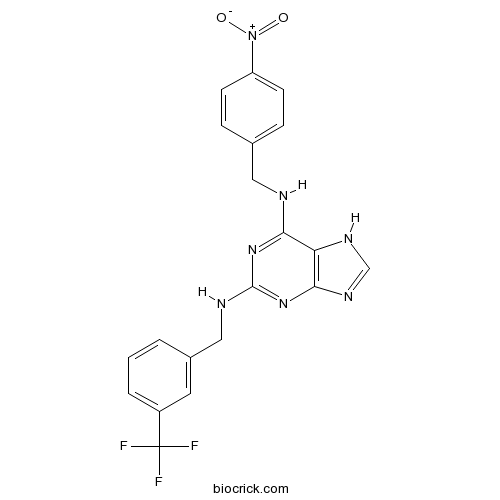
| Chemical Name | 6-N-[(4-nitrophenyl)methyl]-2-N-[[3-(trifluoromethyl)phenyl]methyl]-7H-purine-2,6-diamine | ||
| SMILES | C1=CC(=CC(=C1)C(F)(F)F)CNC2=NC3=C(C(=N2)NCC4=CC=C(C=C4)[N+](=O)[O-])NC=N3 | ||
| Standard InChIKey | DDSBPUYZPWNNGH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H16F3N7O2/c21-20(22,23)14-3-1-2-13(8-14)10-25-19-28-17(16-18(29-19)27-11-26-16)24-9-12-4-6-15(7-5-12)30(31)32/h1-8,11H,9-10H2,(H3,24,25,26,27,28,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reversible inositol hexakisphosphate kinase (IP6K) inhibitor (IC50 = 0.47 μM for inhibition of InsP7 formation). Also inhibits inositol 1,4,5-trisphosphate 3-kinase (IP3K) (IC50 = 10.2 μM). Binds to the ATP binding site of IP3K (Ki = 4.3 μM). |

TNP Dilution Calculator

TNP Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2554 mL | 11.277 mL | 22.554 mL | 45.108 mL | 56.385 mL |
| 5 mM | 0.4511 mL | 2.2554 mL | 4.5108 mL | 9.0216 mL | 11.277 mL |
| 10 mM | 0.2255 mL | 1.1277 mL | 2.2554 mL | 4.5108 mL | 5.6385 mL |
| 50 mM | 0.0451 mL | 0.2255 mL | 0.4511 mL | 0.9022 mL | 1.1277 mL |
| 100 mM | 0.0226 mL | 0.1128 mL | 0.2255 mL | 0.4511 mL | 0.5639 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CJ 033466
Catalog No.:BCC7562
CAS No.:519148-48-2
- Tasisulam
Catalog No.:BCC4407
CAS No.:519055-62-0
- Dehydrohautriwaic acid
Catalog No.:BCN7586
CAS No.:51905-84-1
- 4-(N-Methyl)-aminoantipyrine
Catalog No.:BCC8652
CAS No.:519-98-2
- Sulochrin
Catalog No.:BCN6959
CAS No.:519-57-3
- Maclurin
Catalog No.:BCN5651
CAS No.:519-34-6
- Ellipticine
Catalog No.:BCC7665
CAS No.:519-23-3
- Benzoylecgonine
Catalog No.:BCN1909
CAS No.:519-09-5
- Matrine
Catalog No.:BCN5650
CAS No.:519-02-8
- Pizotifen Malate
Catalog No.:BCC4825
CAS No.:5189-11-7
- Axillarin
Catalog No.:BCN8102
CAS No.:5188-73-8
- 7-O-Methyleriodictyol
Catalog No.:BCN5648
CAS No.:51857-11-5
- 5-Aminoindole
Catalog No.:BCC8735
CAS No.:5192-03-0
- Schaftoside
Catalog No.:BCN2343
CAS No.:51938-32-0
- Dehydroespeletone
Catalog No.:BCN5652
CAS No.:51995-99-4
- Spironolactone
Catalog No.:BCC4366
CAS No.:52-01-7
- Prednisolone Acetate
Catalog No.:BCC4831
CAS No.:52-21-1
- Thio-TEPA
Catalog No.:BCC5354
CAS No.:52-24-4
- Morphine hydrochloride
Catalog No.:BCC6368
CAS No.:52-26-6
- H-D-Pen-OH
Catalog No.:BCC3307
CAS No.:52-67-5
- Lynestrenol
Catalog No.:BCC9014
CAS No.:52-76-6
- Haloperidol
Catalog No.:BCC4909
CAS No.:52-86-8
- H-Cys-OH
Catalog No.:BCC2902
CAS No.:52-90-4
- 6-Methoxyluteolin
Catalog No.:BCN3613
CAS No.:520-11-6
Small molecular probe as selective tritopic sensor of Al(3+), F(-) and TNP: Fabrication of portable prototype for onsite detection of explosive TNP.[Pubmed:28366208]
Anal Chim Acta. 2017 May 1;965:111-122.
Schiff base organic compound (SOC) has been prepared as a tritopic chemosensor for selective sensing by fluorescence signalling towards ions like Al(3+), F(-) and explosive molecule like TNP. In general, fluorescence like photophysical property has been used for selective detection of analyte where Al(3+) and F(-) show turn-on fluorescence signal at different wavelengths (nm) however, quenching was found with TNP. As a consequence, the chemosensor has become a selective sensor for Al(3+), F(-) and TNP. Reversibility of fluorescence responses for Al(3+) and F(-) are observed in presence of ammonium nitrate and H(+) respectively. Similar to the detection of TNP, the detection of explosive like NO3(-) salts is also essential from homeland security point of view. In the present work, the finding of reversible sequential fluorescence response can be promoted for fabrication of next generation AND-NOT-OR-NAND-XOR-XNOR-NOR based complex logic circuit which is applicable in photonics, security and other fields including inorganic and material science. In the case of TNP recognition, the pathway mainly depends on non-covalent interaction (quenching constant: 4.4 x 10(5) M(-1)) which is even better than the recently reported materials. Detection limit for Al(3+), F(-) and TNP is 1 muM, 3 muM and 500 nM respectively. DFT-D3 has been carried out to explore the hostcdots, three dots, centeredguest interaction along with the structure-property correlation of the present host-guest system. All three guest analytes have been detected inside the living cell at a certain level and in its consequence, the successful in vitro recognition ability of the SOCs inside human cell line HeLa has been explored too. In real time stepping, an easy to operate and an economically affordable pocket prototype has also been fabricated for on spot detection of TNP like explosive.
Multiple functional therapeutic effects of TnP: A small stable synthetic peptide derived from fish venom in a mouse model of multiple sclerosis.[Pubmed:28235052]
PLoS One. 2017 Feb 24;12(2):e0171796.
The pathological condition of multiple sclerosis (MS) relies on innate and adaptive immunity. New types of agents that beneficially modify the course of MS, stopping the progression and repairing the damage appear promising. Here, we studied TNP, a small stable synthetic peptide derived from fish venom in the control of inflammation and demyelination in experimental autoimmune encephalomyelitis as prophylactic treatment. TNP decreased the number of the perivascular infiltrates in spinal cord, and the activity of MMP-9 by F4/80+ macrophages were decreased after different regimen treatments. TNP reduces in the central nervous system the infiltration of IFN-gamma-producing Th1 and IL-17A-producing Th17 cells. Also, treatment with therapeutic TNP promotes the emergence of functional Treg in the central nervous system entirely dependent on IL-10. Therapeutic TNP treatment accelerates the remyelination process in a cuprizone model of demyelination. These findings support the beneficial effects of TNP and provides a new therapeutic opportunity for the treatment of MS.
A fluorescent probe based on nitrogen doped graphene quantum dots for turn off sensing of explosive and detrimental water pollutant, TNP in aqueous medium.[Pubmed:28262581]
Spectrochim Acta A Mol Biomol Spectrosc. 2017 Jun 5;180:37-43.
This paper reports the carbonization assisted green approach for the fabrication of nitrogen doped graphene quantum dots (N-GQDs). The obtained N-GQDs displayed good water dispersibility and stability in the wide pH range. The as synthesized N-GQDs were used as a fluorescent probe for the sensing of explosive 2,4,6-trinitrophenol (TNP) in aqueous medium based on fluorescence resonance energy transfer (FRET), molecular interactions and charge transfer mechanism. The quenching efficiency was found to be linear in proportion to the TNP concentration within the range of 0-16muM with detection limit (LOD) of 0.92muM. The presented method was successfully applied to the sensing of TNP in tap and lake water samples with satisfactory results. Thus, N-GQDs were used as a selective, sensitive and turn off fluorescent sensor for the detection of perilous water contaminant i.e. TNP.
Characterization of a selective inhibitor of inositol hexakisphosphate kinases: use in defining biological roles and metabolic relationships of inositol pyrophosphates.[Pubmed:19208622]
J Biol Chem. 2009 Apr 17;284(16):10571-82.
Inositol hexakisphosphate kinases (IP6Ks) phosphorylate inositol hexakisphosphate (InsP(6)) to yield 5-diphosphoinositol pentakisphosphate (5-[PP]-InsP(5) or InsP(7)). In this study, we report the characterization of a selective inhibitor, N(2)-(m-(trifluoromethy)lbenzyl) N(6)-(p-nitrobenzyl)purine (TNP), for these enzymes. TNP dose-dependently and selectively inhibited the activity of IP6K in vitro and inhibited InsP(7) and InsP(8) synthesis in vivo without affecting levels of other inositol phosphates. TNP did not inhibit either human or yeast Vip/PPIP5K, a newly described InsP(6)/InsP(7) 1/3-kinase. Overexpression of IP6K1, -2, or -3 in cells rescued TNP inhibition of InsP(7) synthesis. TNP had no effect on the activity of a large number of protein kinases, suggesting that it is selective for IP6Ks. TNP reversibly reduced InsP(7)/InsP(8) levels. TNP in combination with genetic studies was used to implicate the involvement of two pathways for synthesis of InsP(8) in yeast. TNP induced a fragmented vacuole phenotype in yeast, consistent with inhibition of Kcs1, a Saccharomyces cerevisiae IP6K. In addition, it also inhibited insulin release from Min6 cells in a dose-dependent manner further implicating InsP(7) in this process. TNP thus provides a means of selectively and rapidly modulating cellular InsP(7) levels, providing a new and versatile tool to study the biological function and metabolic relationships of inositol pyrophosphates.


