Methyl ferulateCAS# 22329-76-6 |
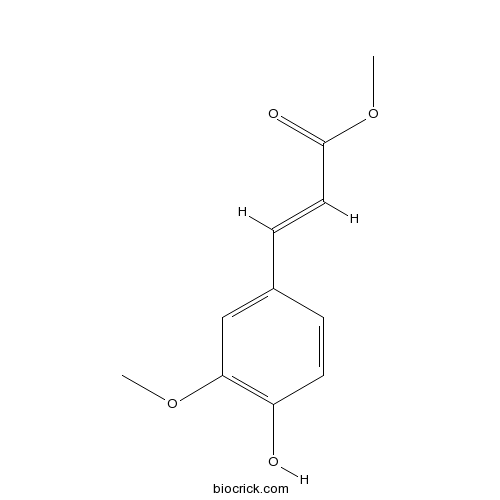
- Ferulic Acid Methyl Ester
Catalog No.:BCN9073
CAS No.:2309-07-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 22329-76-6 | SDF | Download SDF |
| PubChem ID | 5357283 | Appearance | Oil |
| Formula | C11H12O4 | M.Wt | 208.2 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Synonyms | 2309-07-1;Methyl Ferulate;Methyl 4-Hydroxy-3-Methoxycinnamate; Ferulic Acid Methylester; Trans-Methylferulate | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate | ||
| SMILES | COC1=C(C=CC(=C1)C=CC(=O)OC)O | ||
| Standard InChIKey | AUJXJFHANFIVKH-GQCTYLIASA-N | ||
| Standard InChI | InChI=1S/C11H12O4/c1-14-10-7-8(3-5-9(10)12)4-6-11(13)15-2/h3-7,12H,1-2H3/b6-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Methyl ferulate is a possible inhibitor of the mitogen activated phosphor kinase pathway. 2. Methyl ferulate could be a potential anti-inflammatory agent isolated for the first time in medicinal plant S. tuberosa. 3. Methyl ferulate has promising anthelmintic activity against Haemonchus contortus. |
| Targets | IL Receptor | TNF-α | IFN-γ | COX | JNK | NO | p38MAPK | Antifection |

Methyl ferulate Dilution Calculator

Methyl ferulate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8031 mL | 24.0154 mL | 48.0307 mL | 96.0615 mL | 120.0768 mL |
| 5 mM | 0.9606 mL | 4.8031 mL | 9.6061 mL | 19.2123 mL | 24.0154 mL |
| 10 mM | 0.4803 mL | 2.4015 mL | 4.8031 mL | 9.6061 mL | 12.0077 mL |
| 50 mM | 0.0961 mL | 0.4803 mL | 0.9606 mL | 1.9212 mL | 2.4015 mL |
| 100 mM | 0.048 mL | 0.2402 mL | 0.4803 mL | 0.9606 mL | 1.2008 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Platycodigenin
Catalog No.:BCN3183
CAS No.:22327-82-8
- Evodol
Catalog No.:BCN5059
CAS No.:22318-10-1
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- Inolitazone
Catalog No.:BCC1652
CAS No.:223132-37-4
- SEA0400
Catalog No.:BCC1941
CAS No.:223104-29-8
- Finasteride acetate
Catalog No.:BCC4204
CAS No.:222989-99-3
- Noladin ether
Catalog No.:BCC5756
CAS No.:222723-55-9
- SZL P1-41
Catalog No.:BCC8004
CAS No.:222716-34-9
- Chysin A
Catalog No.:BCN2020
CAS No.:22269-11-0
- ACBC
Catalog No.:BCC6584
CAS No.:22264-50-2
- Antidesmone
Catalog No.:BCN5058
CAS No.:222629-77-8
- Bromocriptine mesylate
Catalog No.:BCC6642
CAS No.:22260-51-1
- 9-Hydroxy-alpha-lapachone
Catalog No.:BCN5060
CAS No.:22333-58-0
- Grandiflorenic acid
Catalog No.:BCN4670
CAS No.:22338-67-6
- Grandifloric acid
Catalog No.:BCN4669
CAS No.:22338-69-8
- Polygalacic acid
Catalog No.:BCN5898
CAS No.:22338-71-2
- FG2216
Catalog No.:BCC6402
CAS No.:223387-75-5
- GLP-2 (human)
Catalog No.:BCC5891
CAS No.:223460-79-5
- ONO 4817
Catalog No.:BCC2375
CAS No.:223472-31-9
- YM 58483
Catalog No.:BCC7817
CAS No.:223499-30-7
- CPA inhibitor
Catalog No.:BCC1500
CAS No.:223532-02-3
- Tiadinil
Catalog No.:BCC8070
CAS No.:223580-51-6
- K-115 free base
Catalog No.:BCC5501
CAS No.:223645-67-8
- Collagen proline hydroxylase inhibitor-1
Catalog No.:BCC1495
CAS No.:223663-32-9
Caffeoyl and coumaroyl derivatives from Acacia cochliacantha exhibit ovicidal activity against Haemonchus contortus.[Pubmed:28414046]
J Ethnopharmacol. 2017 May 23;204:125-131.
ETHNOPHARMACOLOGY RELEVANCE: Acacia cochliacantha is a small tree whose foliage is traditionally used in Mexico for treatment of kidney pain, gastrointestinal illnesses and to kill intestinal parasites. In recent decades, the study of vegetal extracts has offered other possible alternatives for the control of Haemonchus contortus. Considering that this nematode affects dramatically the health and productivity of small ruminants, the aim of this study was to identify the anthelmintic compounds from A. cochliacantha hydro-alcoholic extract (HA-E) through an ovicidal test. MATERIAL AND METHODS: In vitro egg hatch assay was conducted to determinate the anthelmintic effects of a HA-E (60g). Liquid-liquid ethyl acetate/water extraction gave two fractions (EtOAc-F, 1.92g; Aq-F; 58.1g). The less polar compounds from ethyl acetate fraction were extracted by addition of dichloromethane offering a precipitate phase (Mt-F, 1.25g) and a soluble mixture (DCMt-F 1.15g). All fractions were evaluated for ovicidal activity obtaining the egg hatching inhibition (EHI, 0.07-25mg/mL). Ivermectin (0.5mg/mL) was used as a reference drug (positive control), and distilled water, 2.5% DMSO and 2% methanol were used as negative controls. The isolated compounds from the most active fractions were subjected to spectroscopic ((1)H NMR) Spectrometric (MS) and UV HPLC analysis in order to identify the bioactive compounds. RESULTS: The less polar treatments (AcOEt-F, DCMt-F, DCMt-P) showed the highest ovicidal activities (98-100% EHI; at 0.62-1.56mg/mL) and the major compounds found in these fractions were identified as caffeoyl and coumaroyl derivatives, including caffeic acid (1), p-coumaric acid (2), ferulic acid (3), methyl caffeate (4), methyl-p-coumarate (5), Methyl ferulate (6) and quercetin. In case of the less active fractions (Aq-F, Mt-F) were constituted principally by glycosylated flavonoids. CONCLUSION: These results show that caffeoyl and coumaroyl derivatives from Acacia cochliacantha leaves had promising anthelmintic activity against Haemonchus contortus. This leguminous may offer an alternative source for the control of gastrointestinal nematodes of small ruminants.
In vitro and in vivo toxicological evaluations of methyl ferulate, methyl p-coumarate, and pulegone 1,2-epoxide.[Pubmed:26067677]
Pharm Biol. 2016;54(3):523-9.
CONTEXT: Toxicological screening of natural compounds for medicinal purposes. OBJECTIVES: The objective of this study is to evaluate the toxicity of Methyl ferulate (MF), methyl p-coumarate (MpC), and pulegone 1,2-epoxide (PE) with in vitro and in vivo assays. MATERIALS AND METHODS: The in vitro toxicity of MF, MpC, and PE was assessed at a concentration of 10 mg/ml with the Ames assay using two strains of Salmonella typhimurium TA98 and TA100. Human red blood cells (RBC) were used to determine the hemolytic activity of these compounds. The cytotoxicity of above compounds was determined with brine shrimp lethality bioassay (BSLB) at the concentrations of 0.1-20 mg/ml. While dermal and ocular irritation studies were conducted on healthy rabbits (n = 8) for 96 and 12 h post-topical application of test compounds, respectively. RESULTS: PE produced 6-8% hemolysis of RBCs at all the tested concentrations while MF and MpC produced 10-5% hemolysis up to 20 mg/ml, and 50-85% hemolysis at concentrations of 40 and 80 mg/ml, respectively. The Ames assay indicated that MF, MpC, and PE were non-mutagenic as the test values were not significantly higher as compared with background values of the assay. BSLB suggested the lethal concentration (LC50) values of MF, MpC, and PE as 4.38, 6.74, and 25.91 mg/ml, respectively. In vivo ocular and dermal irritation scores of MF, MpC, and PE were comparable with ethanol (control) in rabbits indicating the non-irritant nature of these natural compounds. CONCLUSION: The present studies suggest that these compounds are non-toxic/non-irritant and might be used for medicinal purposes.
Anti-inflammatory activity of methyl ferulate isolated from Stemona tuberosa Lour.[Pubmed:25312145]
Asian Pac J Trop Med. 2014 Sep;7S1:S327-31.
OBJECTIVE: To evaluate the anti-inflammatory activity of Methyl ferulate (MF) isolated from the roots of Stemona tuberosa (S. tuberosa) Lour (Stemonaceae) in lipopolysaccharide activated macrophage cells. METHODS: Methanol extracts of a root powder of S. tuberosa were prepared for isolation of a potential anti-inflammatory agent using ultrasound extraction combined with repeated chromatography on silica gel. After the quantitative analyses, anti-inflammatory activity of the isolated compound was evaluated by measurement of cytokine release, NO generation, expression of cyclooxygenase-2 and phosphorylation of mitogen activated protein kinases including p38 and c-Jun NH2-terminal kinase using quantitative kits and Western blotting with specific antibodies. RESULTS: The isolation process yielded a potential anti-inflammatory compound with a purity level of 99% determined by high performance liquid chromatography. The compound was identified as MF by using nuclear magnetic resonance. MF strongly inhibited the release of pro-inflammatory cytokines from macrophages, including IL-6, TNFalpha, IFNgamma, yet it did not affect the anti-inflammatory cytokine IL-10. Phosphorylation of p38 and c-Jun NH2-terminal kinase were clearly reduced in MF-treated macrophages stimulated with lipopolysaccharide. cyclooxygenase-2 expression and NO generation by macrophages were also suppressed when the cells were treated with MF. CONCLUSIONS: The data suggested that MF is a possible inhibitor of the mitogen activated phosphor kinase pathway and could be a potential anti-inflammatory agent isolated for the first time in medicinal plant S. tuberosa.


