MizoribineCAS# 50924-49-7 |
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Proflavine Hemisulfate
Catalog No.:BCC4707
CAS No.:1811-28-5
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- NSC 146109 hydrochloride
Catalog No.:BCC2410
CAS No.:59474-01-0
- Oxaliplatin
Catalog No.:BCC3932
CAS No.:61825-94-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 50924-49-7 | SDF | Download SDF |
| PubChem ID | 4213 | Appearance | Powder |
| Formula | C9H13N3O6 | M.Wt | 259.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NSC 289637; HE 69 | ||
| Solubility | DMSO : ≥ 35 mg/mL (135.02 mM) *"≥" means soluble, but saturation unknown. | ||
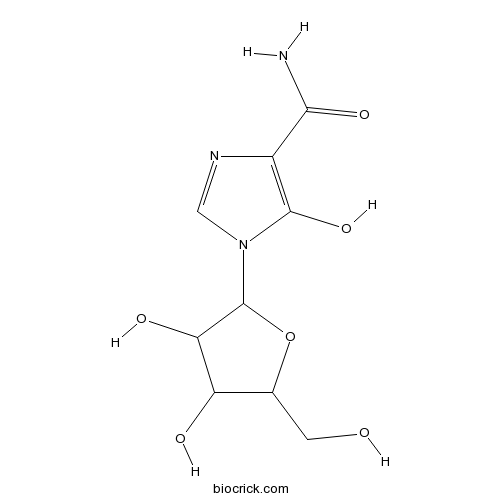
| Chemical Name | 1-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-hydroxyimidazole-4-carboxamide | ||
| SMILES | C1=NC(=C(N1C2C(C(C(O2)CO)O)O)O)C(=O)N | ||
| Standard InChIKey | HZQDCMWJEBCWBR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H13N3O6/c10-7(16)4-8(17)12(2-11-4)9-6(15)5(14)3(1-13)18-9/h2-3,5-6,9,13-15,17H,1H2,(H2,10,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Mizoribine(NSC 289637; HE 69; β-Bredinin) is an immunosuppressive agents (IC50=100 uM) that inhibit the proliferation of lymphocytes selectively, via inhibition of IMPDH.
IC50 Value:
Target: IMPDH
in vitro: Unlike azathioprine, Mizoribine is not taken up by nucleic acids in the cell. Instead, after phosphorylation MZR-5 -monophosphate inhibits GMP synthesis by the antagonistic blocking of IMPDH (Ki = 10(-8)M) and GMP- synthetase (Ki =10(-5) M) [1]. Pretreatment of cells with MZR partially, but significantly, attenuates the expression of monocyte chemoattractant protein (MCP)-1 mRNA and protein, whereas the poly IC-induced expressions for the other functional molecules, such as CCL5, fractalkine and IL-8 were not influenced by MZR treatment [2].
in vivo:MZR 150 mg was administered once a day. After 6 months, the remission rate was 72.7% (2 subjects achieved complete remission, and 9 partial remission). After 3 and 6 months, significant reductions (p < 0.01) were obtained in 24-h proteinuria (g/day) [3]. References: | |||||

Mizoribine Dilution Calculator

Mizoribine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8577 mL | 19.2886 mL | 38.5773 mL | 77.1545 mL | 96.4432 mL |
| 5 mM | 0.7715 mL | 3.8577 mL | 7.7155 mL | 15.4309 mL | 19.2886 mL |
| 10 mM | 0.3858 mL | 1.9289 mL | 3.8577 mL | 7.7155 mL | 9.6443 mL |
| 50 mM | 0.0772 mL | 0.3858 mL | 0.7715 mL | 1.5431 mL | 1.9289 mL |
| 100 mM | 0.0386 mL | 0.1929 mL | 0.3858 mL | 0.7715 mL | 0.9644 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Mizoribine is a immunosuppressive agents (IC50=100 uM) that inhibit the proliferation of lymphocytes selectively, via inhibition of IMPDH.
- Bombinakinin M
Catalog No.:BCC5904
CAS No.:509151-65-9
- Boc-D-Arg(NO2)-OH
Catalog No.:BCC2610
CAS No.:50913-12-7
- 2-Amino-3,5-dibromobenzaldehyde
Catalog No.:BCC8523
CAS No.:50910-55-9
- IRAK-1-4 Inhibitor I
Catalog No.:BCC1659
CAS No.:509093-47-4
- 1beta-Hydroxytorilin
Catalog No.:BCN7095
CAS No.:509078-16-4
- Taiwanhomoflavone B
Catalog No.:BCN5624
CAS No.:509077-91-2
- Nemorensine
Catalog No.:BCN2099
CAS No.:50906-96-2
- Toxyloxanthone D
Catalog No.:BCN3070
CAS No.:50906-62-2
- Arteannuin B
Catalog No.:BCN5623
CAS No.:50906-56-4
- Mitraphylline
Catalog No.:BCC8213
CAS No.:509-80-8
- Strychnine phosphate
Catalog No.:BCC8257
CAS No.:509-42-2
- Napellonine
Catalog No.:BCN2536
CAS No.:509-24-0
- Verminoside
Catalog No.:BCN5625
CAS No.:50932-19-9
- Carminomycin
Catalog No.:BCC6379
CAS No.:50935-04-1, 39472-31-6
- Anacrotine
Catalog No.:BCN2057
CAS No.:5096-49-1
- Crotanecine
Catalog No.:BCN1963
CAS No.:5096-50-4
- Canadine
Catalog No.:BCN5626
CAS No.:5096-57-1
- N-Methylcoclaurine
Catalog No.:BCN7079
CAS No.:5096-70-8
- 16-Methoxystrychnidin-10-One
Catalog No.:BCN8472
CAS No.:5096-72-0
- 7ACC1
Catalog No.:BCC5553
CAS No.:50995-74-9
- Pronethalol hydrochloride
Catalog No.:BCC5678
CAS No.:51-02-5
- Procaine HCl
Catalog No.:BCC5072
CAS No.:51-05-8
- Benzimidazole
Catalog No.:BCC8847
CAS No.:51-17-2
- Fluorouracil (Adrucil)
Catalog No.:BCC2135
CAS No.:51-21-8
Efficacy of mizoribine and prednisolone combination therapy in adult patients with IgA vasculitis.[Pubmed:28255645]
Rheumatol Int. 2017 Aug;37(8):1387-1393.
Immunoglobulin (Ig)A vasculitis (IgAV), formerly known as Henoch-Schonlein purpura, is one of the most common vasculitis caused by an IgA-mediated immune complex. It occurs most frequently in childhood and less commonly in adulthood. As for the treatment of IgAV in adults, there are few studies dealing with the administration and efficacy of intravenous pulse steroid therapy or combination therapy using prednisolone (PSL) and immunosuppressive drugs. Mizoribine (MZB) is a newly developed immunosuppressive drug with few adverse effects; however, there are currently few studies using MZB in adult patients with IgAV. In this study, we evaluated the efficacy of MZB combined with a course of PSL in adult patients with IgAV. Five patients with adult onset IgAV were enrolled in the study. All patients received oral PSL (initial dose 30-50 mg/day), and MZB was administered orally at a single morning dose of 150 mg. We investigated the clinical manifestations and prognosis of these patients receiving the combination therapy of MZB and PSL retrospectively. All patients showed complete or partial remission of proteinuria and microscopic hematuria with the combination therapy of MZB and PSL. Furthermore, no significant adverse effects were observed. Although this study had an uncontrolled small group, our results indicate that the combination of MZB with PSL could be a possible new treatment for adult patients with IgAV.
Enhancement of the antiviral activity against caprine herpesvirus type 1 of Acyclovir in association with Mizoribine.[Pubmed:28235707]
Res Vet Sci. 2017 Apr;111:120-123.
Caprine herpesvirus 1 (CpHV-1) infection in goats is responsible for genital lesions resembling the lesions induced by herpesvirus 2 in humans (HHV-2). The immunosuppressive drug Mizoribine (MIZ) is able to increase the antiviral activity of Acyclovir (ACV) against herpesvirus infections, raising interesting perspectives on new combined therapeutic strategies. In this study the anti-CpHV-1 activity in vitro of ACV alone or in combination with MIZ was evaluated. ACV (100mug/ml) displayed an antiviral effect on CpHV-1 replication. This inhibitory effect was higher when ACV (100mug/ml) was used in association with MIZ (20mug/ml). Other combinations of ACV and MIZ in various concentrations were not as effective as ACV 100mug/ml/MIZ 20mug/ml. These findings suggest that the association of ACV and MIZ is potentially useful for treatment of genital infection by herpesviruses.
Hyperuricemia and Acute Renal Failure in Renal Transplant Recipients Treated With High-Dose Mizoribine.[Pubmed:28104163]
Transplant Proc. 2017 Jan - Feb;49(1):73-77.
BACKGROUND: Hyperuricemia is a common adverse event frequently found in renal transplant recipients with Mizoribine (MZ). Hyperuricemia itself will be a cause of renal dysfunction, and renal dysfunction also will be a cause of hyperuricemia simultaneously. This study investigates frequency of hyperuricemia and renal failure in renal transplant recipients treated with high-dose MZ. PATIENTS AND METHODS: From December 2007 to October 2015, there was a total of 32 living related renal transplant recipients treated with high-dose MZ. Of the 32 patients, 28 were treated with urate-lowering medications. RESULTS: One patient received allopurinol (AP) and 13 patients received benzbromarone (BB). For 6 of them, their urate-lowering medications were converted to febuxostat (FX) form AP or BB. In the remaining 14 patients, FX was administered from the beginning. In 2 cases of ABO-incompatible living related renal transplant recipients who were maintained with high-dose MZ and BB, severe hyperuricemia and acute renal failure occurred. One patient was a 48-year-old man, and his creatinine (Cr) level increased to 8.14 mg/dL and his serum uric acid (UA) was 24.6 mg/dL. Another patient was a 57-year-old man, and his Cr level increased to 3.59 mg/dL and his UA was 13.2 mg/dL. In both cases Cr and UA were improved, and no finding of acute rejection and drug toxicity was observed in graft biopsy specimens. BB was switched to FX and discontinuance or reduction of MZ was done. CONCLUSION: Combination of MZ and BB has the risk of acute renal dysfunction after renal transplantation. Latent renal dysfunction should be watched for in renal transplant recipients receiving high-dose MZ.


