N-Methyl-1-deoxynojirimycinCAS# 69567-10-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 69567-10-8 | SDF | Download SDF |
| PubChem ID | 92381.0 | Appearance | Powder |
| Formula | C7H15NO4 | M.Wt | 177.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
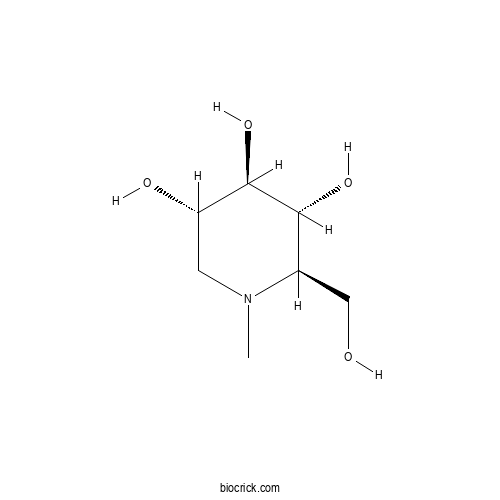
| Chemical Name | (2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine-3,4,5-triol | ||
| SMILES | CN1CC(C(C(C1CO)O)O)O | ||
| Standard InChIKey | AAKDPDFZMNYDLR-XZBKPIIZSA-N | ||
| Standard InChI | InChI=1S/C7H15NO4/c1-8-2-5(10)7(12)6(11)4(8)3-9/h4-7,9-12H,2-3H2,1H3/t4-,5+,6-,7-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

N-Methyl-1-deoxynojirimycin Dilution Calculator

N-Methyl-1-deoxynojirimycin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6433 mL | 28.2167 mL | 56.4334 mL | 112.8668 mL | 141.0835 mL |
| 5 mM | 1.1287 mL | 5.6433 mL | 11.2867 mL | 22.5734 mL | 28.2167 mL |
| 10 mM | 0.5643 mL | 2.8217 mL | 5.6433 mL | 11.2867 mL | 14.1084 mL |
| 50 mM | 0.1129 mL | 0.5643 mL | 1.1287 mL | 2.2573 mL | 2.8217 mL |
| 100 mM | 0.0564 mL | 0.2822 mL | 0.5643 mL | 1.1287 mL | 1.4108 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Monoethyl fumaric acid
Catalog No.:BCX0632
CAS No.:2459-05-4
- Uric acid
Catalog No.:BCX0631
CAS No.:69-93-2
- 1,1,1,1-Kestohexose
Catalog No.:BCX0630
CAS No.:62512-19-0
- Creatinine
Catalog No.:BCX0629
CAS No.:60-27-5
- Blestriarene A
Catalog No.:BCX0628
CAS No.:126721-53-7
- (+)-Balanophonin
Catalog No.:BCX0627
CAS No.:215319-47-4
- γ-sanshool
Catalog No.:BCX0626
CAS No.:78886-65-4
- Yibeinoside A
Catalog No.:BCX0625
CAS No.:98985-24-1
- 4-Methoxy-1,2-benzenediol
Catalog No.:BCX0624
CAS No.:3934-97-2
- 1-Deoxyforskolin
Catalog No.:BCX0623
CAS No.:72963-77-0
- 2,3-Dimethoxybenzoic acid
Catalog No.:BCX0622
CAS No.:1521-38-6
- 6-Deoxy-D-glucose
Catalog No.:BCX0621
CAS No.:7658-08-4
- Ocimene
Catalog No.:BCX0634
CAS No.:13877-91-3
- Manzamine A
Catalog No.:BCX0635
CAS No.:104196-68-1
- Crocetin methylester
Catalog No.:BCX0636
CAS No.:25368-09-6
- Lucidin Omega-Methyl Ether
Catalog No.:BCX0637
CAS No.:79560-36-4
- Schiarisanrin A
Catalog No.:BCX0638
CAS No.:130252-41-4
- Palmitoleic acid methyl ester
Catalog No.:BCX0639
CAS No.:1120-25-8
- Gymnoside IX
Catalog No.:BCX0640
CAS No.:898827-00-4
- 2-O-β-D-Glucopyranosyl-L-ascorbic acid
Catalog No.:BCX0641
CAS No.:562043-82-7
- Isokadsuranin
Catalog No.:BCX0642
CAS No.:82467-52-5
- 8-Epi-Loganic acid-6'-O-β-D-glucoside
Catalog No.:BCX0643
CAS No.:176226-39-4
- L-xylose
Catalog No.:BCX0644
CAS No.:609-06-3
- Apigenin-6-C-β-D-xylopyranosyl-8-C-α-L-arabinopyranoside
Catalog No.:BCX0645
CAS No.:85700-46-5
Novel active compounds and the anti-diabetic mechanism of mulberry leaves.[Pubmed:36278175]
Front Pharmacol. 2022 Oct 5;13:986931.
Mulberry (Morus alba L.) leaves have long been considered beneficial in traditional Chinese medicine to treat infectious and internal diseases. Recently studies have discovered that the mulberry leaf's total flavonoids (MLF) display excellent hypoglycemia properties. However, the active ingredients and their molecular mechanisms are still uncharacterized. In this study, we explored the hypoglycemic effects of MLF and mulberry leaf polysaccharides (MLP) on ob/ob mice, an animal model of type 2 diabetes mellitus (T2DM), compared with Ramulus Mori (Sangzhi) alkaloid (RMA). Network pharmacology was employed to identify the potential available targets and active compounds of MLF and RMA against hyperglycemia. Molecular docking, an insulin-resistant cell model and qPCR were employed to verify the antidiabetic activity of the critical compounds and the gene expression profiles of the top molecular targets. Here, the results showed that MLF and MLP improved glucose uptake in insulin-resistant hepatocytes. MLF, MLP and RMA alleviated insulin resistance and glucose intolerance in ob/ob mice. Unlike MLF and MLP, RMA administration did not influence the accumulation of intrahepatic lipids. Network pharmacology analysis revealed that morusin, kuwanon C and morusyunnansin L are the main active compounds of MLF and that they amend insulin resistance and glycemia via the PI3K- Akt signaling pathway, lipid and atherosclerosis pathways, and the AGE-RAGE signaling pathway. Moreover, 1-deoxynojirimycin (DNJ), fagomine (FA), and N-Methyl-1-deoxynojirimycin are the primary active ingredients of RMA and target carbohydrate metabolism and regulate alpha-glucosidase activity to produce a potent anti-diabetic effect. The molecular docking results indicated that morusin, kuwanon C and morusyunnansin L are the critical bioactive compounds of MLF. They had high affinities with the key targets adenosine A1 receptor (ADORA1), AKT serine/threonine kinase 1 (AKT1), peroxisome proliferator-activated receptor gamma (PPARgamma), and glycogen synthase kinase 3 beta (GSK3beta), which play crucial roles in the MLF-mediated glucose-lowering effect. Additionally, morusin plays a role in amending insulin resistance of hepatocytes by repressing the expression of the ADORA1 and PPARG genes. Our results shed light on the mechanism behind the glucose-lowering effects of MLF, suggesting that morusin, kuwanon C, and morusyunnansin L might be promising drug leads for the management of T2DM.
The aqueous extract of Fridericia chica grown in northern Colombia ameliorates toxicity induced by Tergitol on Caenorhabditis elegans.[Pubmed:33626396]
Comp Biochem Physiol C Toxicol Pharmacol. 2021 Jun;244:109026.
The aqueous extract of fallen leaves from Fridericia chica (Bonpl.) L.G. Lohmann is utilized as a remedy in communities at northern Colombia. Traditional uses include wound healing, gastrointestinal inflammation, leukemia and psoriasis, among others. The aims of this research were to evaluate the potential of the aqueous extract of fallen leaves of F. chica (AEFchica) to inhibit ethoxylated nonylphenol (Tergitol)-induced toxicity in Caenorhabditis elegans; and to identify its main components. The pharmacological properties of AEFchica was evaluated using a Tergitol-induced toxicity model in Caenorhabditis elegans. Lethality, locomotion, reproduction, and DAF-16 nuclear translocation were quantified. The chemical composition of AEFchica was carried out using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. AEFchica induced very little lethality on C. elegans (5.6%) even at high concentrations (10,000 mug/mL). The extract had no effect on locomotion impairing induced by ethoxylated nonylphenol. However, AEFchica (1000 mug/mL) abrogated Tergitol-induced mortality, recovering up to 53.3% of the nematodes from lethality induced by 10 mM Tergitol. Similarly, it also blocked Tergitol-dependent reproduction inhibition (82.1% recovery), as well as DAF-16 nuclear translocation (>95%), suggesting a prominent role on oxidative stress control. The chemical analysis indicated the presence of a great variety of molecules with known antioxidant, metabolic and immune modulator properties, such as hydroxylated methoxy flavones, N-Methyl-1-deoxynojirimycin, and rehmaionoside A. In short, the aqueous extract of F. chica protects C. elegans from the deleterious effects of Tergitol on lethality, reproduction and oxidative stress involving DAF-16-mediated pathway. This extract is a promising source of bioactive phytochemicals for multi-target pharmacological purposes.
A UPLC-MS/MS method for simultaneous determination of 1-deoxynojirimycin and N-methyl-1-deoxynojirimycin in rat plasma and its application in pharmacokinetic and absolute bioavailability studies.[Pubmed:29179061]
J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Jan 1;1072:205-210.
A specific, sensitive, rapid, precise, and reliable UPLC-MS/MS-based method was designed for the first time for the simultaneous determination of 1-deoxynojirimycin (DNJ) and N-Methyl-1-deoxynojirimycin (N-CH(3)-DNJ) in rat plasma. Miglitol was served as the internal standard (IS). An MN-NUCLEODUR HILIC column was assessed to separate the two compounds by isocratic elution using acetonitrile: water with 0.05% formic acid and 6.5mM ammonium acetate (72:28, v/v) at a flow rate of 0.4mL/min. A triple quadrupole mass spectrometer was operated in the positive ionization mode using multiple reaction monitoring (MRM), and it was employed to determine transitions of m/z 164.1-->110.1, 178.1-->100.1, and 208.1-->146.1 for DNJ, N-CH(3)-DNJ, and IS, respectively. The method of the two constituents was validated and the results were acceptable. The absolute bioavailability of DNJ and N-CH(3)-DNJ in rats was 50+/-9% and 62+/-24%, respectively. The method was then successfully used for the first time to study the pharmacokinetic behavior and absolute bioavailability of DNJ and N-CH(3)-DNJ in rats after intravenous (10mg/kg) and oral administration (80mg/kg). The results of this study might provide more information on preclinical pharmacokinetics and a solid basis for assessing the clinical efficacy of DNJ and N-CH(3)-DNJ.
Biological study of the angiogenesis inhibitor N-(8-(3-ethynylphenoxy)octyl-1-deoxynojirimycin.[Pubmed:20565474]
Chem Biol Drug Des. 2010 Jun;75(6):570-7.
The alpha-glucosidase inhibitors N-Methyl-1-deoxynojirimycin (MDNJ) and castanospermine have been shown to inhibit angiogenesis. A hybrid of 1-deoxynojirimycin (DNJ) and an aryl-1,2,3-triazole, which inhibits both an alpha-glucosidase and methionine aminopeptidase-2 (MetAP2), displayed properties associated with inhibition of angiogenesis (Bioorg. Med. Chem., 16, 2008, 6333-7). The biological evaluation of a structural analogue N-(8-(3-ethynylphenoxy)octyl-1-deoxynojirimycin is described herein. Although this alkyne derivative did not inhibit MetAP2, it inhibited a bacterial alpha-glucosidase, altered bovine aortic endothelial cell (BAEC) surface oligosaccharide expression and inhibited BAEC proliferation by inducing G1 phase cell cycle arrest. Experiments showed G1 arrest was attributable to the alpha-glucosidase inhibitor inducing an increase in p27(Kip1) expression and high phosphorylation of ERK1/2 without a reduction in cyclin D1. The DNJ derivative (0.1 mM) prevented capillary tube formation from bovine aortic endothelial cells, whereas DNJ or other analogues were unable to inhibit tube formation at the same concentration. Stress fiber assembly in bovine aortic endothelial cells was abolished, and BAEC migration was inhibited indicating the inhibition of tube formation by this derivative is partially a result of a reduction in cell motility. The agent also caused a reduction in secretion of MMP-2 from bovine aortic endothelial cells. Therefore, the new alpha-glucosidase inhibitor has a different mechanism by which it inhibits angiogenesis in vitro when compared with deoxynojirimycin, the deoxynojirimycin -triazole hybrid, N-Methyl-1-deoxynojirimycin and castanospermine.
1-Deoxynojirimycin derivatives from the marine sponge Lendenfeldia chondrodes.[Pubmed:17080688]
J Antibiot (Tokyo). 2006 Aug;59(8):507-11.
Two 1-deoxynojirimycin derivatives, 1-deoxynojirimycin-6-phosphate (1) and N-Methyl-1-deoxynojirimycin-6-phosphate (2) were isolated from an aqueous extract of Micronesian marine sponge Lendenfeldia chondrodes for the first time as natural products. Structures of these compounds were assigned on the basis of their spectral data and chemical degradation.
N-methyl-1-deoxynojirimycin (MOR-14), an alpha-glucosidase inhibitor, markedly improves postischemic left ventricular dysfunction.[Pubmed:11766064]
Heart Vessels. 2000;15(6):268-73.
We examined whether pharmacological inhibition of glycogenolysis by N-Methyl-1-deoxynojirimycin (MOR-14), a new compound which reduces the glycogenolytic rate by inhibiting the alpha-1,6-glucosidase activity of the glycogen-debranching enzyme, can protect the heart against postischemic left ventricular dysfunction. The hearts of male Sprague-Dawley rats were excised, and perfused on a Langendorff apparatus with Krebs-Henseleit solution with a gas mixture of 95% O2 and 5% CO2. The hearts were paced at 320 beats/min except during the ischemia. Left ventricular developed pressure (LVDP, mmHg), +/-dP/dt (mmHg/s), and coronary flow (ml/min) were continuously monitored. All hearts were perfused for a total of 120 min including a 30-min preischemic period followed by a 30-min episode of global ischemia and 60 min reperfusion. with or without 0.5 or 2 mM of MOR-14 during the 30-min preischemic period or the first 30 min of reperfusion. In another series of experiments, the myocardial content of glycogen and lactate was measured during the 30-min episode of ischemia in groups treated with and without 2mM of MOR-14. Preischemic but not postischemic treatment with MOR-14 significantly improved LVDP and +/-dP/dt without altering coronary flow during reperfusion in a dose-dependent manner. MOR-14 significantly preserved the glycogen content and significantly attenuated the lactate accumulation during the 30-min episode of ischemia. Preischemic treatment with MOR-14 is protective against postischemic left ventricular dysfunction through the inhibition of glycogenolysis in the isolated rat heart.
Combination of N-methyl-1-deoxynojirimycin and ischemic preconditioning markedly reduces the size of myocardial infarcts in rabbits.[Pubmed:11446504]
Jpn Circ J. 2001 Jul;65(7):673-7.
N-Methyl-1-deoxynojirimycin (NMDN), an a-glucosidase inhibitor, reduces myocardial infarct size by reducing the glycogenolytic rate through inhibition of the alpha-1,6-glucosidase of glycogen-debranching enzyme in the heart, in addition to possessing an antihyperglycemic action by blocking alpha-1,4-glucosidase in the intestine. Ischemic preconditioning (PC), which markedly reduces the size of the myocardial infarct, is known to reduce the activity of phosphorylase and reduce the glycogenolytic rate. Therefore, it was hypothesized that a combination of pharmacological inhibition of glycogenolysis by an alpha-1,6-glucosidase inhibitor, NMDN, and PC could markedly reduce myocardial infarct size more than NMDN or PC alone. Japanese white rabbits without collateral circulation were subjected to a 30-min coronary occlusion followed by 48-h reperfusion. The infarct sizes as a percentage of area at risk were significantly reduced by pre-ischemic treatment with either 100mg/kg of NMDN or PC of 5 min ischemia and 5 min reperfusion alone (15.9+/-2.0%, n=8, and 10.3+/-1.2%, n=8, respectively) as compared with the control (43.9+/-2.2%, n=8). However, the combination of 100mg/kg of NMDN and PC significantly reduced the infarct size (4.9+/-1.2, n=8) compared with NMDN or PC alone. Another 40 rabbits, also given 100mg of NMDN, PC, NMDN+PC or saline before ischemia (n=10 in each group), were killed for biochemical analysis after 30 min of ischemia. NMDN and PC preserved the glycogen content and attenuated the lactate accumulation, respectively, as compared with the control. However, the combination of NMDN and PC preserved significantly more glycogen and significantly reduced lactate accumulation than either NMDN or PC alone. The combination of NMDN and PC markedly reduced the myocardial infarct size more than either process alone. The marked preservation of glycogen and marked attenuation of lactate accumulation by the combination of NMDN and PC suggest that the mechanism for this effect of NMDN+PC is related to the inhibition of glycogenolysis.
Role of protein kinase C in the reduction of infarct size by N-methyl-1-deoxynojirimycin, an alpha-1,6-glucosidase inhibitor.[Pubmed:11429386]
Br J Pharmacol. 2001 Jul;133(5):635-42.
Preischaemic treatment with N-Methyl-1-deoxynojirimycin (MOR-14), an alpha-1,6-glucosidase inhibitor, attenuates glycogenolysis and lactate accumulation during ischaemia and markedly reduces infarct size in rabbit hearts. In the present study, we have investigated whether protein kinase C (PKC), a principal mediator of ischaemic preconditioning, is also involved in the cardioprotective effect of MOR-14. To assess the effect of PKC inhibition on infarct size in MOR-14-treated hearts, 38 rabbits were subjected to 30 min of ischaemia followed by 48 h of reperfusion. Infarct size, as a per cent of area at risk, was significantly smaller in rabbits administered 100 mg kg(-1) of MOR-14 10 min before ischaemia (17+/-2%, n=10), than in a control group (46+/-5%, n=10). This beneficial effect of MOR-14 was abolished when 5 mg kg(-1) of chelerythrine, a PKC inhibitor, was given 10 min prior to MOR-14 injection (39+/-4%, n=10), although chelerythrine alone did not alter infarct size (43+/-4%, n=8). Further, chelerythrine had no effect on MOR-14-induced attenuation of glycogen breakdown and lactate accumulation in hearts excised at 30 min of ischaemia. Immunoblot analysis of PKC in homogenates of Langendorff-perfused rabbit hearts revealed that MOR-14 significantly increased levels of PKC-epsilon in the particulate fraction at 20 and 30 min of ischaemia and in the cytosolic fraction at 30 min of ischaemia. Taken as a whole, our data suggest that PKC acts downstream of the inhibition of glycogenolysis by MOR-14 to reduce infarct size. Thus, activation of PKC is a more direct mediator of the cardioprotection afforded by MOR-14 than is inhibition of glycogenolysis.
Effect of inhibitors of glycoprotein processing on cytokine secretion and production in anti CD3-stimulated T cells.[Pubmed:10706401]
Biol Pharm Bull. 2000 Jan;23(1):1-5.
We have investigated the effect of inhibitors of glycoprotein processing on cytokine secretion and production in anti CD3-stimulated T cells to elucidate the role of carbohydrate in the triggering of T cell function. The inhibitors of glycoprotein processing, especially mannnosidase inhibitors, enhanced the anti CD3-induced production of interleukin-2 (IL-2), which is a cytokine without the linkage sequence of N-linked oligosaccharides. On the other hand, N-Methyl-1-deoxynojirimycin (NMdNM, an inhibitor of processing glucosidase 1), 1-deoxynojirimycin (dNM, an inhibitor of processing glucosidase I and II) and bromoconduritol (BCD, an inhibitor of processing glucosidase II) inhibited the secretion of interleukin-4 (IL-4), interferon-gamma (IFN-gamma), or interleukin-5 (IL-5) into culture supernatants of anti CD3-stimulated T cells, which had N-linked oligosaccharides. Mannosidase inhibitors, 1-deoxymannojirimycin (dMAN, an inhibitor of processing mannosidase I) and swainsonine (SWN, an inhibitor of processing mannosidase II) did not inhibit the secretion or production of IL-4, IFN-gamma and IL-5. To confirm the inhibition of N-linked oligosaccharide processing in the cytokines by the above inhibitors, the binding of IFN-gamma to lectins with various sugar-binding specificities was investigated. All inhibitors reduced the binding of IFN-gamma to PHA E4, which had a high affinity to bi- or tri-antennary complex type N-linked oligosaccharides with bisecting N-acetylglucosamine. Similarly, all inhibitors reduced the binding of IFN-gamma to PHA L4, which had high affinity to tri- or tetra-antennary complex type N-linked oligosaccharides with beta1-6-linked branching. SWN and dMAN increased the binding of IFN-gamma to concanavalin A (ConA), which had a high affinity to bi-antennary complex type, hybrid type and high-mannose type N-linked oligosaccharides. These results suggest that the processing inhibitors used here inhibit the N-linked oligosaccharide processing of cytokines, and the inhibition of processing enzyme glucosidases I and II induces a decreased secretion of cytokines with N4-linked oligosaccharides.
N-methyl-1-deoxynojirimycin (MOR-14), an alpha-glucosidase inhibitor, markedly reduced infarct size in rabbit hearts.[Pubmed:9570200]
Circulation. 1998 Apr 7;97(13):1290-7.
BACKGROUND: N-Methyl-1-deoxynojirimycin (MOR-14), an alpha-glucosidase inhibitor, reduces the glycogenolytic rate by inhibiting the alpha-1,6-glucosidase of glycogen-debranching enzyme in the liver, in addition to possessing an antihyperglycemic action by blocking alpha-1,4-glucosidase in the intestine. Because the reduction of the glycogenolytic rate may be one of the mechanisms of myocardial protection in ischemic preconditioning, the compounds inhibiting myocardial alpha-1,6-glucosidase may be protective against ischemic damage. Thus, we investigated whether MOR-14 could inhibit alpha-1,6-glucosidase and reduce the infarct size in rabbit hearts without collateral circulation. METHODS AND RESULTS: MOR-14 dose-dependently decreased the alpha-1,6-glucosidase activity in rabbit heart extract. A tracer study demonstrated the myocardial uptake of a considerable amount of MOR-14 sufficient to fully inhibit alpha-1,6-glucosidase. To assess the infarct size-reducing effect of MOR-14, 54 rabbits were subjected to 30-minute coronary occlusion followed by 48-hour reperfusion. Preischemic treatment with 25, 50, and 100 mg/kg of MOR-14 dose-dependently reduced the infarct size (to 26+/-4%, 19+/-3%, and 14+/-2% of the area at risk, respectively), compared with the saline control (45+/-5%) without altering the blood pressure or heart rate. Another 40 rabbits given 100 mg of MOR-14 or saline 10 minutes before ischemia were euthanized at 10 or 30 minutes of ischemia for biochemical analysis. MOR-14 decreased the alpha-1,6-glucosidase activity to approximately 20% in vivo, reduced the glycogen breakdown, and attenuated the lactate accumulation at both 10 and 30 minutes of ischemia. CONCLUSIONS: Preischemic treatment with MOR-14 preserved glycogen, attenuated the accumulation of lactate, and reduced the myocardial infarct size by 69%. This cardioprotective effect was independent of changes of blood pressure and heart rate or regional blood flow. It may be associated with alpha-1,6-glucosidase inhibition, because MOR-14 markedly decreased the alpha-1,6-glucosidase activity in the heart.
Autotaxin is an N-linked glycoprotein but the sugar moieties are not needed for its stimulation of cellular motility.[Pubmed:7496154]
Melanoma Res. 1995 Aug;5(4):203-9.
Autotaxin is a 125kD autocrine motility factor that stimulates both random and directed motility in producing the human A2058 melanoma cell line. The recently cloned autotaxin has been demonstrated to bind strongly and specifically to concanavalin A (con A). In this study, we show that the oligosaccharide side chains on autotaxin are exclusively asparagine linked, since N-glycosidase F, but not neuraminidase or O-glycosidase, decreases the protein molecular mass to 100-105kD, which is the calculated molecular mass of the deduced autotaxin polypeptide. Furthermore, removal of oligosaccharide side chains by N-glycosidase F can be performed under mild conditions that retain motility-stimulating activity, suggesting that the oligosaccharide side chains are not necessary for autotaxin to activate its receptor. Finally, when melanoma cells are treated with inhibitors of carbohydrate processing, such as N-Methyl-1-deoxynojirimycin, 1-deoxymannojirimycin and swainsonine, they still secrete a motility-stimulating autotaxin. Therefore, the carbohydrate side chains on autotaxin are not necessary to stimulate motility; however, they may still play a role in folding, secretion or maintenance of the active conformation of the protein.


