OcimeneCAS# 13877-91-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13877-91-3 | SDF | Download SDF |
| PubChem ID | 5281553.0 | Appearance | Powder |
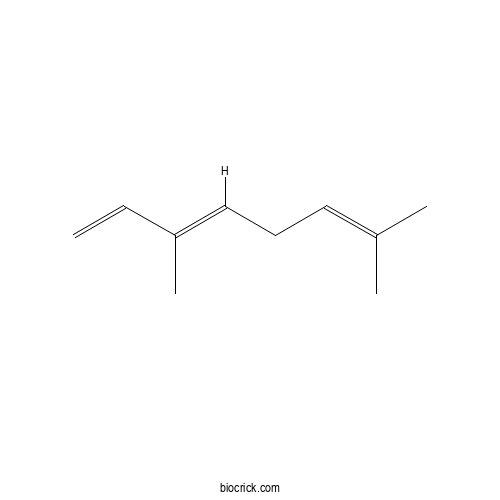
| Formula | C10H16 | M.Wt | 136.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | beta-Ocimene | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3E)-3,7-dimethylocta-1,3,6-triene | ||
| SMILES | CC(=CCC=C(C)C=C)C | ||
| Standard InChIKey | IHPKGUQCSIINRJ-CSKARUKUSA-N | ||
| Standard InChI | InChI=1S/C10H16/c1-5-10(4)8-6-7-9(2)3/h5,7-8H,1,6H2,2-4H3/b10-8+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ocimene Dilution Calculator

Ocimene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.3405 mL | 36.7026 mL | 73.4053 mL | 146.8105 mL | 183.5132 mL |
| 5 mM | 1.4681 mL | 7.3405 mL | 14.6811 mL | 29.3621 mL | 36.7026 mL |
| 10 mM | 0.7341 mL | 3.6703 mL | 7.3405 mL | 14.6811 mL | 18.3513 mL |
| 50 mM | 0.1468 mL | 0.7341 mL | 1.4681 mL | 2.9362 mL | 3.6703 mL |
| 100 mM | 0.0734 mL | 0.367 mL | 0.7341 mL | 1.4681 mL | 1.8351 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N-Methyl-1-deoxynojirimycin
Catalog No.:BCX0633
CAS No.:69567-10-8
- Monoethyl fumaric acid
Catalog No.:BCX0632
CAS No.:2459-05-4
- Uric acid
Catalog No.:BCX0631
CAS No.:69-93-2
- 1,1,1,1-Kestohexose
Catalog No.:BCX0630
CAS No.:62512-19-0
- Creatinine
Catalog No.:BCX0629
CAS No.:60-27-5
- Blestriarene A
Catalog No.:BCX0628
CAS No.:126721-53-7
- (+)-Balanophonin
Catalog No.:BCX0627
CAS No.:215319-47-4
- γ-sanshool
Catalog No.:BCX0626
CAS No.:78886-65-4
- Yibeinoside A
Catalog No.:BCX0625
CAS No.:98985-24-1
- 4-Methoxy-1,2-benzenediol
Catalog No.:BCX0624
CAS No.:3934-97-2
- 1-Deoxyforskolin
Catalog No.:BCX0623
CAS No.:72963-77-0
- 2,3-Dimethoxybenzoic acid
Catalog No.:BCX0622
CAS No.:1521-38-6
- Manzamine A
Catalog No.:BCX0635
CAS No.:104196-68-1
- Crocetin methylester
Catalog No.:BCX0636
CAS No.:25368-09-6
- Lucidin Omega-Methyl Ether
Catalog No.:BCX0637
CAS No.:79560-36-4
- Schiarisanrin A
Catalog No.:BCX0638
CAS No.:130252-41-4
- Palmitoleic acid methyl ester
Catalog No.:BCX0639
CAS No.:1120-25-8
- Gymnoside IX
Catalog No.:BCX0640
CAS No.:898827-00-4
- 2-O-β-D-Glucopyranosyl-L-ascorbic acid
Catalog No.:BCX0641
CAS No.:562043-82-7
- Isokadsuranin
Catalog No.:BCX0642
CAS No.:82467-52-5
- 8-Epi-Loganic acid-6'-O-β-D-glucoside
Catalog No.:BCX0643
CAS No.:176226-39-4
- L-xylose
Catalog No.:BCX0644
CAS No.:609-06-3
- Apigenin-6-C-β-D-xylopyranosyl-8-C-α-L-arabinopyranoside
Catalog No.:BCX0645
CAS No.:85700-46-5
- Phytosphingosine
Catalog No.:BCX0646
CAS No.:554-62-1
Flowering Ocimum gratissimum intercropped in tea plantations attracts and reduces Apolygus lucorum populations.[Pubmed:38587057]
Pest Manag Sci. 2024 Apr 8.
BACKGROUND: Apolygus lucorum is one of the most important piercing-sucking insect pests of the tea plant In this study, we assessed the attractiveness of basil plants to A. lucorum and the effectiveness of Ocimum gratissimum L. in the control of A. lucorum. The control efficiency of main volatile chemicals emitted from O. gratissimum flowers was also evaluated. RESULTS: Among seven basil varieties, O. gratissimum was more attractive to A. lucorum adults and was selected as a trap plant to assess its attractiveness to A. lucorum and effects on natural enemies in tea plantations. The population density of A. lucorum on trap strips of O. gratissimum in tea plantations was significantly higher than that on tea at 10-20 m away from the trap strips. Intercropping O. gratissimum with tea plants, at high-density significantly reduced A. lucorum population levels. Eucalyptol, limonene, beta-Ocimene, and linalool were the four dominant components in the O. gratissimum flower volatiles, and their emissions showed a gradual upward trend over the sampling period. Olfactometer assays indicated that eucalyptol and dodecane showed attraction to A. lucorum. High numbers of A. lucorum were recorded on limonene, eucalyptol, and myrcene-baited yellow sticky traps in field trials in which 11 dominant volatiles emitted by O. gratissimum flowers were evaluated. CONCLUSION: Our research indicated that the aromatic plant O. gratissimum and its volatiles could attract A. lucorum and planting O. gratissimum has the potential as a pest biocontrol method to manipulate A. lucorum populations in tea plantations. (c) 2024 Society of Chemical Industry.
Biological activity of essential oils from Ferulago angulata and Ferula assa-foetida against food-related microorganisms (antimicrobial) and Ephestia kuehniella as a storage pest (insecticidal); an in vitro and in silico study.[Pubmed:38565381]
Fitoterapia. 2024 Mar 31;175:105937.
Misuse of synthetic pesticides and antimicrobials in agriculture and the food industry has resulted in food contamination, promoting resistant pests and pathogen strains and hazards for humanity and the environment. Therefore, ever-increasing concern about synthetic chemicals has stimulated interest in eco-friendly compounds. Ferulago angulata (Schltdl.) Boiss. and Ferula assa-foetida L., as medicinal species with restricted natural distribution and unknown biological potential, aimed at investigation of their essential oil (EO) biological properties, were subjected. Z-beta-Ocimene and Z-1-Propenyl-sec-butyl disulfide molecules were identified as the major composition of the essential oil of the fruits of F. angulata and F. assa-foetida, respectively. In vitro antimicrobial activity and membrane destruction investigation by scanning electron microscopy imaging illustrated that F. angulata EO had potent antibacterial activity. Besides, the EOs of both plants exhibited significant anti-yeast activity against Candida albicans. In relation to insecticidal activity, both EOs indicated appropriate potential against Ephestia kuehniella; however, the F. assa-foetida EO had more toxicity on the studied pest. Among several insecticidal-related targets, acetylcholinesterase was identified as the main target of EO based on the molecular docking approach. Hence, in line with in vitro results, in silico evaluation determined that F. assa-foetida has a higher potential for inhibiting acetylcholinesterase and, consequently, better insecticide properties. Overall, in addition to the antioxidant properties of both EO, F. angulata EO could serve as an effective prevention against microbial spoilage and foodborne pathogens, and F. assa-foetida EO holds promise as a multi-purpose and natural biocide for yeast contamination and pest management particularly against E. kuehniella.
The Essential Oil Compositions of Ambrosia acanthicarpa Hook., Artemisia ludoviciana Nutt., and Gutierrezia sarothrae (Pursh) Britton & Rusby (Asteraceae) from the Owyhee Mountains of Idaho.[Pubmed:38543021]
Molecules. 2024 Mar 20;29(6):1383.
As part of our interest in the volatile phytoconstituents of aromatic plants of the Great Basin, we have obtained essential oils of Ambrosia acanthicarpa (three samples), Artemisia ludoviciana (12 samples), and Gutierrezia sarothrae (six samples) from the Owyhee Mountains of southwestern Idaho. Gas chromatographic analyses (GC-MS, GC-FID, and chiral GC-MS) were carried out on each essential oil sample. The essential oils of A. acanthicarpa were dominated by monoterpene hydrocarbons, including alpha-pinene (36.7-45.1%), myrcene (21.6-25.5%), and beta-phellandrene (4.9-7.0%). Monoterpene hydrocarbons also dominated the essential oils of G. sarothrae, with beta-pinene (0.5-18.4%), alpha-phellandrene (2.2-11.8%), limonene (1.4-25.4%), and (Z)-beta-Ocimene (18.8-39.4%) as major components. The essential oils of A. ludoviciana showed wide variation in composition, but the relatively abundant compounds were camphor (0.1-61.9%, average 14.1%), 1,8-cineole (0.1-50.8%, average 11.1%), (E)-nerolidol (0.0-41.0%, average 6.8%), and artemisia ketone (0.0-46.1%, average 5.1%). This is the first report on the essential oil composition of A. acanthicarpa and the first report on the enantiomeric distribution in an Ambrosia species. The essential oil compositions of A. ludoviciana and G. sarothrae showed wide variation in composition in this study and compared with previous studies, likely due to subspecies variation.
Aroma Characteristics of Green Huajiao in Sichuan and Chongqing Area Using Sensory Analysis Combined with GC-MS.[Pubmed:38540826]
Foods. 2024 Mar 9;13(6):836.
Green huajiao has a unique flavor and is widely used in cooking as an edible spice. In this study, the intensity of overall aroma and aroma attributes of seven green huajiao samples from the Sichuan and Chongqing regions were evaluated using a dynamic dilution olfactometer and ranking descriptive analysis (RDA) technology. The volatile compounds and major aroma components were determined by GC-MS in combination with odor activity value (OAV) analysis. The partial least squares regression (PLSR) model was further used to identify the key aromas contributing to the aroma sensory attributes. Seven green huajiao samples were categorized into three groups: (1) huajiao samples from Liangshan have a strong intensity of pungent, floral and herbal aromas and a medium-high intensity of sweet aroma, and the key contributing aroma compounds were alpha-pinene, sabinene, beta-pinene, myrcene, Ocimene and linalool; (2) huajiao samples from Panzhihua and Hongya have a strong intensity of citrusy, lemony and minty aromas, and the key contributing aroma compound was linalool; and (3) the huajiao sample from the Chongqing region was categorized into a separate group and was characterized by a medium-high intensity of green, minty and sweet aromas, and the main aroma compounds are Ocimene, citronellal and alpha-terpineol. These results provide useful basic data for evaluating the aroma quality and analyzing the key aroma characteristics of green huajiao in the Sichuan and Chongqing regions.
Seseli tortuosum L. subsp. tortuosum Essential Oils and Their Principal Constituents as Anticancer Agents.[Pubmed:38475524]
Plants (Basel). 2024 Feb 28;13(5):678.
Seseli tortuosum L. subsp. tortuosum, belonging to the Apiaceae family, is a species that grows in Europe, mainly in the Mediterranean regions. The history of its application in traditional medicine highlights its various biological properties. Trying to explore the phytochemistry and pharmacological aspects of this species, the essential oils (EOs) extracted from flowers, stems, and roots of a locally wild accession, never previously investigated, growing in Sicily, Italy, were investigated. The chemical composition of all EOs, obtained by the hydrodistillation method, was evaluated by GC-MS. The most abundant class of all investigated samples was that of monoterpene hydrocarbons (79.98-91.21%) with p-cymene, alpha-pinene, beta-pinene, and beta-Ocimene as major compounds. These EOs, and their main components, were tested for their possible anticancer activity. Obtained data provided evidence that among the different EOs tested, at the dose of 100 mug/mL, those extracted from stems and roots were particularly effective, already at 24 h of treatment, in reducing the cell viability of 42% and 95%, respectively, in HCT116 colon cancer cell line. These EOs also exerted a remarkable cytotoxic effect that was accompanied by morphological changes represented by cell shrinkage as well as a reduction in residual cell population. Differently, modest effects were found when EOs extracted from flowers were tested in the same experimental conditions. The evaluation of the phytocompounds mainly represented in the EOs extracted from different parts of the plant and tested in a range of concentrations between 20 and 200 mug/mL, revealed that alpha-pinene, beta-pinene, and p-cymene exerted only modest effects on cell viability. Differently, a remarkable effect was found when beta-Ocimene, the most abundant phytocomponent in EOs from roots, was tested on colon cancer cells. This phytocompound, among those identified in EOs from Seseli tortuosum L. subsp. tortuosum, was found to be the most effective in reducing colon cancer cell viability with IC(50) = 64.52 mug/mL at 24 h of treatment. All together, these data suggest that beta-Ocimene could be responsible for the effects observed in colon cancer cells.
Evaluation of the Antimicrobial Activity of Geraniol and Selected Geraniol Transformation Products against Gram-Positive Bacteria.[Pubmed:38474462]
Molecules. 2024 Feb 21;29(5):950.
Both geraniol and the products of its transformation, thanks to their beneficial properties, find a variety of applications in cosmetics. Due to their antioxidant and moisturizing properties, these compounds can be added to skin care products such as face creams, lotions, oils, and masks. In addition, these compounds show some antibacterial and antifungal properties, making them suitable for application in skin care products to help fight against bacteria or fungi. This study determined the antimicrobial activity of geraniol and the compounds which were formed during its transformation in relation to selected Gram-positive bacteria, and the preliminary assessment was made whether these compounds can act as ingredients of preparations with potential antimicrobial activity in the treatment of various human diseases (for example diseases of the skin, digestive system, or urinary tract). In addition, this work presents studies on the microbiological purity of cream samples obtained with different contents of geraniol and its transformation products (contents of the tested compounds: 0.5%, 1.5%, 2.5%, 4%, 8%, and 12%). Antibacterial activity tests were performed using the disc diffusion method against Gram-positive cocci, including the reference strains Staphylococcus aureus ATCC 29213 and Enterococcus faecalis ATCC 29212, and against the clinical strains Staphylococcus aureus MRSA, Staphylococcus epidermidis, Enterococcus faecalis VRE VanB, Enterococcus faecium VRE VanA, and Enterococcus faecium VRE VanB. The most active ingredient against bacteria of the Staphylococcus genus was citral, followed by linalool and then geraniol. During our tests, in the case of bacteria of the Enterococcus genus, citral also showed the highest activity, but linalool, Ocimenes, and geraniol showed a slightly lower activity. Moreover, this study examined the microbiological purity of cream samples obtained with various contents of geraniol and its transformation products. In the tests of the microbiological purity of cream samples, no growth of aerobic bacteria and fungi was found, which proves the lack of microbiological contamination of the obtained cosmetic preparations. On this basis, it was assessed that these compounds have preservative properties in the prepared creams. The addition of the analyzed compounds also had influence on the durability of the creams and had no effect on the change in their consistency, did not negatively affect the separation of phases during storage, and even had a positive effect on organoleptic sensations by enriching the smell of the tested samples.
EsigPBP3 Was the Important Pheromone-Binding Protein to Recognize Male Pheromones and Key Eucalyptus Volatiles.[Pubmed:38474187]
Int J Mol Sci. 2024 Mar 3;25(5):2940.
Pheromone-binding proteins (PBPs) are specific odorant-binding proteins that can specifically recognize insect pheromones. Through transcriptional analysis of the antennae of adult Endoclita signifer, EsigPBP3 was discovered and identified, and EsigPBP3 was found to be highly expressed in the antennae of male moths. Based on the binding characteristics and ability of EsigPBP3, we can find the key ligands and binding site to consider as a target to control the key wood bore E. signifier. In this study, the fluorescence competitive binding assays (FCBA) showed that EsigPBP3 had a high binding affinity for seven key eucalyptus volatiles. Molecular docking analysis revealed that EsigPBP3 had the strongest binding affinity for the sexual pheromone component, (3E,7E)-4,7,11-trimethyl-1,3,7,10-dodecatetraene. Furthermore, same as the result of FCBA, the EsigPBP3 exhibited high binding affinities to key eucalyptus volatiles, eucalyptol, alpha-terpinene, (E)-beta-Ocimene, (-)-beta-pinene, and (-)-alpha-pinene, and PHE35, MET7, VAL10, PHE38, ILE52, and PHE118 are key sites. In summary, EsigPBP3 exhibits high binding affinity to male pheromones and key volatile compounds and the crucial binding sites PHE35, MET7, VAL10, PHE38, ILE52, and PHE118 can act as targets in the recognition of E. signifier pheromones.
A diterpene synthase from the sandfly Lutzomyia longipalpis produces the pheromone sobralene.[Pubmed:38470919]
Proc Natl Acad Sci U S A. 2024 Mar 19;121(12):e2322453121.
The phlebotomine sandfly, Lutzomyia longipalpis, a major vector of the Leishmania parasite, uses terpene pheromones to attract conspecifics for mating. Examination of the L. longipalpis genome revealed a putative terpene synthase (TPS), which-upon heterologous expression in, and purification from, Escherichia coli-yielded a functional enzyme. The TPS, termed LlTPS, converted geranyl diphosphate (GPP) into a mixture of monoterpenes with low efficiency, of which beta-Ocimene was the major product. (E,E)-farnesyl diphosphate (FPP) principally produced small amounts of (E)-beta-farnesene, while (Z,E)- and (Z,Z)-FPP yielded a mixture of bisabolene isomers. None of these mono- and sesquiterpenes are known volatiles of L. longipalpis. Notably, however, when provided with (E,E,E)-geranylgeranyl diphosphate (GGPP), LlTPS gave sobralene as its major product. This diterpene pheromone is released by certain chemotypes of L. longipalpis, in particular those found in the Ceara state of Brazil. Minor diterpene components were also seen as products of the enzyme that matched those seen in a sandfly pheromone extract.
The Role of Ocimene in Decreasing alpha-Synuclein Aggregation using Rotenone-induced Rat Model.[Pubmed:38409725]
Cent Nerv Syst Agents Med Chem. 2024 Feb 23.
BACKGROUND: Parkinson's disease is defined by the loss of dopaminergic neurons in the midbrain of substantia nigra associated with Lewy bodies. The precise mechanism is not yet entirely understood. OBJECTIVE: The study aims to determine whether Ocimene has antiparkinsonian activity by reducing alpha-Synuclein aggregation levels in the brains of rotenone-induced rat models. METHODS: 36 male rats were used for six groups, with six animals in each group. Vehicle, control (rotenone, 2.5 mg/kg, i.p), standard (L-Dopa, 10 mg/kg, i.p), Test drug of low dose (66.66 mg/kg, i.p), medium dose (100 mg/kg, i.p), and high dose (200 mg/kg, i.p) were administered to the rats. The open field, actophotometer, hanging wire, and catalepsy tests were used to assess the rat's motor performance. The expressions of biomarkers such as AchE, D2 Receptor, and alpha- Synuclein were evaluated, and their level of expression in the brain samples was checked using ELISA. Histopathological analysis was also carried out to determine the degree of neuron degeneration in the brain samples. RESULTS: The open field test showed significant anxiety levels, whereas test groups showed fewer anxiety levels but increased motor activity. The biochemical tests revealed that rotenonetreated rats had higher levels of AchE, but Ocimene-treated rats had a significant decrease in AchE levels. The test drug-treated rats also expressed high levels of D2 receptors. In Ocimenetreated rats, alpha-Synuclein aggregation was reduced, however, in rotenone-treated rats' brain samples, higher clumps of alpha-Synuclein were observed. CONCLUSION: Ocimene has neuroprotective properties. As a result, this essential oil might be helpful as a therapeutic treatment for Parkinson's disease.
Identification of Volatile Compounds and Terpene Synthase (TPS) Genes Reveals ZcTPS02 Involved in beta-Ocimene Biosynthesis in Zephyranthes candida.[Pubmed:38397175]
Genes (Basel). 2024 Jan 30;15(2):185.
Zephyranthes candida is a frequently cultivated ornamental plant containing several secondary metabolites, including alkaloids, flavonoids, and volatile organic compounds (VOCs). However, extensive research has been conducted only on non-VOCs found in the plant, whereas the production of VOCs and the molecular mechanisms underlying the biosynthesis of terpenes remain poorly understood. In this study, 17 volatile compounds were identified from Z. candida flowers using gas chromatography-mass spectrometry (GC-MS), with 16 of them being terpenoids. Transcriptome sequencing resulted in the identification of 17 terpene synthase (TPS) genes; two TPS genes, ZcTPS01 and ZcTPS02, had high expression levels. Biochemical characterization of two enzymes encoded by both genes revealed that ZcTPS02 can catalyze geranyl diphosphate (GPP) into diverse products, among which is beta-Ocimene, which is the second most abundant compound found in Z. candida flowers. These results suggest that ZcTPS02 plays a vital role in beta-Ocimene biosynthesis, providing valuable insights into terpene biosynthesis pathways in Z. candida. Furthermore, the expression of ZcTPS02 was upregulated after 2 h of methyl jasmonate (MeJA) treatment and downregulated after 4 h of the same treatment.
Non-targeted metabolomic analysis reveals the mechanism of quality formation of citrus flower-green tea.[Pubmed:38380915]
J Sci Food Agric. 2024 Feb 21.
BACKGROUND: Citrus flower-green tea (CT) is a scented tea processed from green tea (GT) and fresh citrus flower, which is favored by consumers due to its potential health benefits and unique citrus flavor. This study evaluated the quality of CT and revealed the mechanism of its quality formation. RESULTS: The CT had a significant citrus flavor and a good antioxidant activity, and its sensory quality was superior to that of GT. Headspace solid-phase microextraction-gas chromatography-mass spectrometry (HS-SPME-GC-MS) analysis revealed that the scenting process resulted in a significant increase of alkenes such as beta-pinene, trans-beta-Ocimene, alpha-farnesene, isoterpinolene, and gamma-terpinene, as well as a significant decrease of alcohols such as alpha-terpineol, l-menthol, and linalool in CT in comparison with GT. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis revealed that the levels of flavonoids (such as neohesperidin, hesperidin, tangeritin, hesperetin 5-O-glucoside, and nobiletin) and alkaloids (such as trigonelline and theobromine) in CT increased significantly after scenting process, while the levels of amino acids (such as valine and l-phenylalanine) and organic acids (such as ascorbic acid) decreased significantly. CONCLUSION: These observations showed that the scenting process promoted the absorption of aroma from citrus flowers by GT and the changes in its non-volatile metabolites, leading to the formation of citrus flavor quality in CT. (c) 2024 Society of Chemical Industry.
The effects of different extraction methods on essential oils from orange and tangor: From the peel to the essential oil.[Pubmed:38370058]
Food Sci Nutr. 2023 Oct 25;12(2):804-814.
Citrus fruits are largely consumed due to their unique and pleasant aromas. Citrus hybrids have been developed to enhance their flavors and bioactivities. Citrus aroma depends on the composition of the volatile compounds in citrus essential oils (CEOs), which are mostly located in the peels. During the extraction of CEOs, a specific series of chemical reactions occurred depending on the extraction methods (CP, cold pressing; HD, hydrodistillation), leading to variations in the composition of volatile compounds. In this study, the orange and the tangor which is a hybrid between C. reticulata x C. sinensis were investigated to compare the changes in volatile compounds based on the extraction methods. Results showed that the CP-specific volatile compounds were sesquiterpenes, oxygenated monoterpenes, and fatty acid derivatives, while the HD-specific volatile compounds were terpinyl cation derivatives, limonene, and 4-vinylguaiacol. On the other hand, the contents of some volatile compounds ((E)-Ocimene, alpha-terpinene, and alpha-terpinolene) were affected by the Citrus species rather than by the extraction methods. In particular, during HD, terpinene-4-ol and 4-vinylguiacol, known as off-flavor compounds in citrus juice, were formed more abundantly in the orange than in the tangor. In conclusion, these results provide comprehensive data on essential oils, especially those derived from oranges and tangors, for selecting the appropriate extraction method for obtaining a higher yield and quality of citrus flavor.
Multiomics integrated with sensory evaluations to identify characteristic aromas and key genes in a novel brown navel orange (Citrus sinensis).[Pubmed:38325085]
Food Chem. 2024 Jun 30;444:138613.
'Zong Cheng' navel orange (ZC) is a brown mutant of Lane Late navel orange (LL) and emits a more pleasant odor than that of LL. However, the key volatile compound of this aroma and underlying mechanism remains unclear. In this study, sensory evaluations and volatile profiling were performed throughout fruit development to identify significant differences in sensory perception and metabolites between LL and ZC. It revealed that the sesquiterpene content varied significantly between ZC and LL. Based on aroma extract dilution and gas chromatography-olfactometry analyses, the volatile compound leading to the background aroma of LL and ZC is d-limonene, the orange note in LL was mainly attributed to octanal, whilst valencene, beta-myrcene, and (E)-beta-Ocimene presented balsamic, sweet, and herb notes in ZC. Furthermore, Cs5g12900 and six potential transcription factors were identified as responsible for valencene accumulation in ZC, which is important for enhancing the aroma of ZC.
Polar Molecules Regulating the Regio- and Stereoselectivity of Polymerization of Conjugated Dienes Catalyzed by CGC-Type Rare-Earth Metal Catalysts.[Pubmed:38261808]
Macromol Rapid Commun. 2024 Apr;45(7):e2300653.
Herein, a concise, effective, and scalable strategy is reported that the introduction of polar molecules (PMs) (e.g., anisole (PhOMe), phenetole (PhOEt), 2-methoxynaphthalene (NaphOMe), thioanisole (PhSMe), and N,N-dimethylaniline (PhNMe(2))) as continuously coordinated neutral ligand of cationic active species in situ generated from the constrain-geometry-configuration-type rare-earth metal complexes A-F/Al(i)Bu(3)/[Ph(3)C][B(C(6)F(5))(4)] ternary systems can easily switch the regio- and stereoselectivity of the polymerization of conjugated dienes (CDs, including 2-subsituted CDs such as isoprene (IP) and myrcene (MY), 1,2-disubstituted CD Ocimene (OC), and 1-substituted polar CD 1-(para-methoxyphenyl)-1,3-butadiene (p-MOPB)) from poor selectivities to high selectivities (for IP and MY: 3,4-selectivity up to 99%; for OC: trans-1,2-selectivity up to 93% (mm up to 90%); for p-MOPB: 3,4-syndioselectivity (3,4- up to 99%, rrrr up to 96%)). DFT calculations explain the continuous coordination roles of PMs on the regulation of the regio- and stereoselectivity of the polymerization of CDs. In comparison with the traditional strategies, this strategy by adding some common PMs is easier and more convenient, decreasing the synthetic cost and complex operation of new metal catalyst and cocatalyst. Such regio- and stereoselective regulation method by using PMs is not reported for the coordination polymerization of olefins catalyzed by rare-earth metal and early transition metal complexes.
Communication between undamaged plants can elicit changes in volatile emissions from neighbouring plants, thereby altering their susceptibility to aphids.[Pubmed:38254306]
Plant Cell Environ. 2024 May;47(5):1543-1555.
Plant volatiles play an important role in intra- and interspecific plant communication, inducing direct and indirect defenses against insect pests. However, it remains unknown whether volatile interactions between undamaged cultivars alter host plant volatile emissions and their perception by insect pests. Here, we tested the effects of exposure of a spring barley, Hordeum vulgare L., cultivar, Salome, to volatiles from other cultivars: Fairytale and Anakin. We found that exposing Salome to Fairytale induced a significantly higher emission of trans-beta-Ocimene and two unidentified compounds compared when exposed to Anakin. Aphids were repelled at a higher concentration of trans-beta-Ocimene. Salome exposure to Fairytale had significant repulsive effects on aphid olfactory preference, yet not when Salome was exposed to Anakin. We demonstrate that volatile interactions between specific undamaged plants can induce changes in volatile emission by receiver plants enhancing certain compounds, which can disrupt aphid olfactory preferences. Our results highlight the significant roles of volatiles in plant-plant interactions, affecting plant-insect interactions in suppressing insect pests. This has important implications for crop protection and sustainable agriculture.


