Palmitoleic acid methyl esterCAS# 1120-25-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1120-25-8 | SDF | Download SDF |
| PubChem ID | 643801.0 | Appearance | Powder |
| Formula | C17H32O2 | M.Wt | 268.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
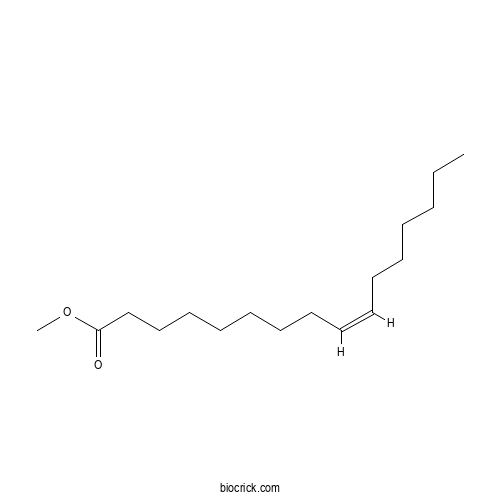
| Chemical Name | methyl (Z)-hexadec-9-enoate | ||
| SMILES | CCCCCCC=CCCCCCCCC(=O)OC | ||
| Standard InChIKey | IZFGRAGOVZCUFB-HJWRWDBZSA-N | ||
| Standard InChI | InChI=1S/C17H32O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17(18)19-2/h8-9H,3-7,10-16H2,1-2H3/b9-8- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Palmitoleic acid methyl ester Dilution Calculator

Palmitoleic acid methyl ester Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7254 mL | 18.6268 mL | 37.2537 mL | 74.5073 mL | 93.1342 mL |
| 5 mM | 0.7451 mL | 3.7254 mL | 7.4507 mL | 14.9015 mL | 18.6268 mL |
| 10 mM | 0.3725 mL | 1.8627 mL | 3.7254 mL | 7.4507 mL | 9.3134 mL |
| 50 mM | 0.0745 mL | 0.3725 mL | 0.7451 mL | 1.4901 mL | 1.8627 mL |
| 100 mM | 0.0373 mL | 0.1863 mL | 0.3725 mL | 0.7451 mL | 0.9313 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Schiarisanrin A
Catalog No.:BCX0638
CAS No.:130252-41-4
- Lucidin Omega-Methyl Ether
Catalog No.:BCX0637
CAS No.:79560-36-4
- Crocetin methylester
Catalog No.:BCX0636
CAS No.:25368-09-6
- Manzamine A
Catalog No.:BCX0635
CAS No.:104196-68-1
- Ocimene
Catalog No.:BCX0634
CAS No.:13877-91-3
- N-Methyl-1-deoxynojirimycin
Catalog No.:BCX0633
CAS No.:69567-10-8
- Monoethyl fumaric acid
Catalog No.:BCX0632
CAS No.:2459-05-4
- Uric acid
Catalog No.:BCX0631
CAS No.:69-93-2
- 1,1,1,1-Kestohexose
Catalog No.:BCX0630
CAS No.:62512-19-0
- Creatinine
Catalog No.:BCX0629
CAS No.:60-27-5
- Blestriarene A
Catalog No.:BCX0628
CAS No.:126721-53-7
- (+)-Balanophonin
Catalog No.:BCX0627
CAS No.:215319-47-4
- Gymnoside IX
Catalog No.:BCX0640
CAS No.:898827-00-4
- 2-O-β-D-Glucopyranosyl-L-ascorbic acid
Catalog No.:BCX0641
CAS No.:562043-82-7
- Isokadsuranin
Catalog No.:BCX0642
CAS No.:82467-52-5
- 8-Epi-Loganic acid-6'-O-β-D-glucoside
Catalog No.:BCX0643
CAS No.:176226-39-4
- L-xylose
Catalog No.:BCX0644
CAS No.:609-06-3
- Apigenin-6-C-β-D-xylopyranosyl-8-C-α-L-arabinopyranoside
Catalog No.:BCX0645
CAS No.:85700-46-5
- Phytosphingosine
Catalog No.:BCX0646
CAS No.:554-62-1
- Genistein 8-C-glucoside
Catalog No.:BCX0647
CAS No.:66026-80-0
- Coniferylaldehydel
Catalog No.:BCX0648
CAS No.:458-36-6
- D-Tartaric acid
Catalog No.:BCX0649
CAS No.:147-71-7
- 4-Ethoxybenzyl alcohol
Catalog No.:BCX0650
CAS No.:6214-44-4
- Dehydrosulphurenic acid
Catalog No.:BCX0651
CAS No.:175615-56-2
Exploring Phytochemical Composition and In Vivo Anti-Inflammatory Potential of Grape Seed Oil from an Alternative Source after Traditional Fermentation Processes: Implications for Phytotherapy.[Pubmed:37570949]
Plants (Basel). 2023 Jul 28;12(15):2795.
This study aimed to analyze the composition of grape seed oil (GSO) derived from an alternative source after traditional fermentation processes and its potential anti-inflammatory effects using an in vivo model of carrageenan-induced inflammation in mice. Gas chromatography high-resolution electron ionization mass spectrometry (GC-HR-EIMS) analysis identified eight main components in the GSO extract, including myristic acid methyl ester, Palmitoleic acid methyl ester, methyl isoheptadecanoate, cis-linoleic acid, oleic acid methyl ester, linoleic acid stereoisomer, linoleic acid ethyl ester, and methyl (6E, 9E, 12E, 15E)-docose-6,9,12,15-tetraenoate. No significant differences were observed in the main fatty acids between commercially available grape seed oil and GSO extract obtained from fermented grape seeds. In the carrageenan-induced inflammation model, treatment with GSO resulted in a significant reduction in paw edema at 180 min, as in the reduction observed with diclofenac treatment. Combined treatment with GSO and diclofenac showed enhanced anti-inflammatory effects. Additionally, GSO exhibited antioxidative effects by decreasing the levels of glutathione (GSH) and malondialdehyde (MDA) in the serum. Chronic treatment with GSO for ten days did not provide a protective effect on inflammation. These findings suggest that GSO could be used as an alternative raw material and could possess anti-inflammatory and antioxidative properties. Further studies are needed to explore its potential therapeutic applications.
Garlic Allelochemical Diallyl Disulfide Alleviates Autotoxicity in the Root Exudates Caused by Long-Term Continuous Cropping of Tomato.[Pubmed:32991155]
J Agric Food Chem. 2020 Oct 21;68(42):11684-11693.
Continuous cropping obstacles seriously affect the sustainable production of tomatoes (Solanum lycopersicum L.). Researchers have found that intercropping with garlic (Allium sativum L.) could alleviate tomato continuous cropping obstacles. Diallyl disulfide (DADS) is the main allelochemical in garlic. However, the mechanism of DADS in alleviating tomato continuous cropping obstacles is still unknown. In this research, aqueous extracts of tomato continuous cropping soil were used to simulate the continuous cropping condition of tomato. Our results showed that DADS increased root activity and chlorophyll content and improved the activity of antioxidant enzymes (superoxide dismutase (SOD), peroxidase (POD), and phenylalanine ammonia-lyase (PAL)) and the metabolism of nonenzymatic antioxidants (glutathione (GSH) and oxidized glutathione (GSSG)) in tomato plants. DADS treatment reduced the content of fatty acid esters in tomato root exudates (e.g., palmitate methyl ester, Palmitoleic acid methyl ester, oleic acid methyl ester) and increased the level of substances such as dibutyl phthalate and 2,2'-methylenebis(6-tert-butyl-4-methylphenol). The higher concentrations of palmitate methyl ester inhibited tomato hypocotyl growth, while oleic acid methyl ester inhibited tomato root growth. Moreover, the application of DADS significantly inhibited the secretion of these esters in the root exudates. Therefore, it suggests that DADS may increase tomato resistance and promote tomato plant growth by increasing root activity and photosynthetic capacity and development to reduce autotoxicity of tomato.


