NKH 477Water-soluble adenylyl cyclase activator CAS# 138605-00-2 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 138605-00-2 | SDF | Download SDF |
| PubChem ID | 444028 | Appearance | Powder |
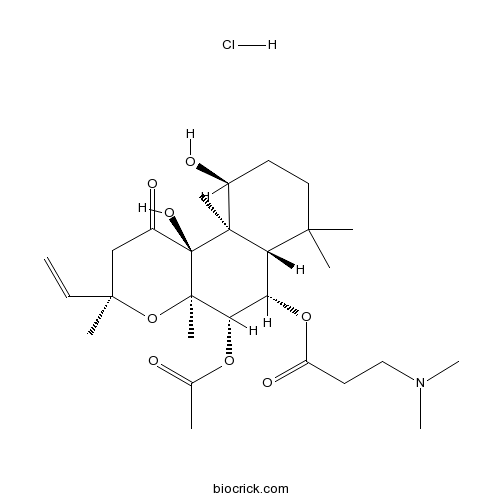
| Formula | C27H44ClNO8 | M.Wt | 546.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Colforsin dapropate hydrochloride, 6-(3-dimethylaminopropionyl)forskolin hydrochloride | ||
| Solubility | Soluble to 40 mM in water | ||
| Chemical Name | [(3R,4aR,5S,6S,6aS,10S,10aR,10bS)-5-acetyloxy-3-ethenyl-10,10b-dihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-5,6,6a,8,9,10-hexahydro-2H-benzo[f]chromen-6-yl] 3-(dimethylamino)propanoate;hydrochloride | ||
| SMILES | CC(=O)OC1C(C2C(CCC(C2(C3(C1(OC(CC3=O)(C)C=C)C)O)C)O)(C)C)OC(=O)CCN(C)C.Cl | ||
| Standard InChIKey | VIRRLEDAYYYTOD-YHEOSNBFSA-N | ||
| Standard InChI | InChI=1S/C27H43NO8.ClH/c1-10-24(5)15-18(31)27(33)25(6)17(30)11-13-23(3,4)21(25)20(35-19(32)12-14-28(8)9)22(34-16(2)29)26(27,7)36-24;/h10,17,20-22,30,33H,1,11-15H2,2-9H3;1H/t17-,20-,21-,22-,24-,25-,26+,27-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Water-soluble analog of forskolin that is a potent activator of adenylyl cyclase; shows some selectivity for cardiac (type V) adenylyl cyclase. Stimulates bronchodilation (EC50 = 32.6 nM) and is an orally active potent positive chronotrope and hypotensive agent in vivo. Also available as part of the PKA. |

NKH 477 Dilution Calculator

NKH 477 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8312 mL | 9.1558 mL | 18.3117 mL | 36.6233 mL | 45.7792 mL |
| 5 mM | 0.3662 mL | 1.8312 mL | 3.6623 mL | 7.3247 mL | 9.1558 mL |
| 10 mM | 0.1831 mL | 0.9156 mL | 1.8312 mL | 3.6623 mL | 4.5779 mL |
| 50 mM | 0.0366 mL | 0.1831 mL | 0.3662 mL | 0.7325 mL | 0.9156 mL |
| 100 mM | 0.0183 mL | 0.0916 mL | 0.1831 mL | 0.3662 mL | 0.4578 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N3PT
Catalog No.:BCC1782
CAS No.:13860-66-7
- 9-Hydroxycanthin-6-one
Catalog No.:BCN3105
CAS No.:138544-91-9
- Tormentic acid
Catalog No.:BCN6198
CAS No.:13850-16-3
- Pomolic acid
Catalog No.:BCN6197
CAS No.:13849-91-7
- 3-Oxopomolic acid
Catalog No.:BCN3485
CAS No.:13849-90-6
- S-(+)-Marmesin
Catalog No.:BCN8288
CAS No.:13849-08-6
- Ro 31-8220 Mesylate
Catalog No.:BCC4999
CAS No.:138489-18-6
- FK 888
Catalog No.:BCC5907
CAS No.:138449-07-7
- A 1070722
Catalog No.:BCC7933
CAS No.:1384424-80-9
- Irbesartan
Catalog No.:BCC2560
CAS No.:138402-11-6
- Tinospinoside C
Catalog No.:BCN6925
CAS No.:1383977-51-2
- Afzelechin-(4alpha->8)-epiafzelechin
Catalog No.:BCN7709
CAS No.:1383627-30-2
- (R)-3-Carboxy-4-hydroxyphenylglycine
Catalog No.:BCC6606
CAS No.:13861-03-5
- Securitinine
Catalog No.:BCN6986
CAS No.:13861-71-7
- LP 20 hydrochloride
Catalog No.:BCC6266
CAS No.:1386928-34-2
- Eugenol rutinoside
Catalog No.:BCN6201
CAS No.:138772-01-7
- Fmoc-D-1-Nal-OH
Catalog No.:BCC3284
CAS No.:138774-49-3
- Fmoc-D-2-Nal-OH
Catalog No.:BCC3290
CAS No.:138774-94-4
- Fmoc-N-Me-Ile-OH
Catalog No.:BCC3214
CAS No.:138775-22-1
- Isomangiferolic acid
Catalog No.:BCN4768
CAS No.:13878-92-7
- Fmoc-D-Thr(tBu)-OH
Catalog No.:BCC3555
CAS No.:138797-71-4
- 8'-Oxo-6-hydroxydihydrophaseic acid
Catalog No.:BCN7046
CAS No.:1388075-44-2
- Pasakbumin B
Catalog No.:BCN2991
CAS No.:138809-10-6
- 5'-Methoxyhexahydrocurcumin
Catalog No.:BCN7049
CAS No.:138870-96-9
Behavioural effects of NKH-477, a forskolin analogue, on locomotion and rearing in rats.[Pubmed:11343575]
Int J Neuropsychopharmacol. 2000 Mar;3(1):27-33.
NKH-477 is a water-soluble analogue of forskolin that stimulates adenylate cyclase and has antidepressant properties in the forced swim model of depression. The effect of chronic NKH-477 on locomotion and rearing and its behavioural interaction with lithium and inositol was tested. NKH-477 (1.5 mg/kg), administered acutely or chronically for 9 d to rats, induced a significant reduction in locomotor behaviour and rearing. In a second experiment, rats were pretreated with chronic dietary lithium, inositol or control diet for 3 wk before being injected with either saline or NKH-477. Inositol had no effect on the decreased locomotion and rearing induced by NKH-477. There was a significant, but transient, interaction between lithium and NKH-477 on rearing on the second and third days of treatment. The lithium group had lower baseline rearing than control or inositol-treated rats, and was not further affected by NKH-477 treatment. This is the first report of a marked effect of this forskolin analogue on locomotion and rearing, with no tolerance in the time frame examined. The interaction between NKH-477 and lithium suggests that the well-documented ability of lithium to inhibit stimulation of the cAMP system is relevant to its behavioural effects; however, the transient nature of this interaction, suggests that other biochemical mechanisms are involved in the behavioural effects of lithium.
Relaxant effects of NKH477, a new water-soluble forskolin derivative, on guinea-pig tracheal smooth muscle: the role of Ca2+-activated K+ channels.[Pubmed:9517396]
Br J Pharmacol. 1998 Feb;123(4):753-61.
1. Mechanisms underlying the bronchorelaxant action of NKH477, a newly developed water-soluble forskolin derivative, were investigated in guinea-pig isolated tracheal smooth muscle. 2. In muscles precontracted with 3 microM histamine, NKH477 (1 nM-1 microM) caused a concentration-dependent decrease of isometric tension, resulting in a complete relaxation at 300 nM. The EC550 for the relaxation was 32.6+/-4.3 nM (n=6). 3. In the presence of 30 or 90 nM iberiotoxin (IbTX), a selective blocker of the large-conductance Ca2+-activated K+ (BK(Ca)) channel, the relaxing action of NKH477 on the histamine-induced contraction was inhibited, giving rise to a parallel shift of the concentration-response curves; the EC50 of NKH477 was increased to 131.4+/-20.4 nM at 30 nM IbTX (n=4), and 125.3+/-12.2 nM at 90 nM IbTX (n=4). 4. Pretreatment of muscles with 30 mM tetraethylammonium (TEA) caused a similar rightward shift of the concentration-response curve to NKH477 with an increase of the EC50 to 139.8+/-18.4 nM (n=5). In contrast, the relaxing action of NKH477 was unaffected by 10 microM glibenclamide, an ATP-sensitive K channel blocker, or by 100 nM apamin, a blocker of small conductance Ca2+-activated K+ channels. 5. In muscles pretreated with 1 microM nifedipine, a blocker of the voltage-dependent Ca2+ channel (VDC), 30-90 nM IbTX did not affect the relaxant effects of NKH477 on the histamine-induced contraction. 6. In muscles precontracted by a K+-rich (40 mM) solution, NKH477 caused only minimal relaxation (19.8+/-1.7%, n=4) even at the highest concentration (1 microM). 7. In experiments to measure the ratio of fura-2 fluorescence signals (R(340/380)) as an index of the intracellular Ca2+ concentration ([Ca2+]i), the application of 100 nM NKH477 or 200 nM isoprenaline to the preparation precontracted by 3 microM histamine resulted in a decrease in [Ca2+]i in association with a decrease in tension. The reduction of [Ca2+]i and tension by NKH477 was 47.0+/-5.6% and 62.8+/-7.0%, respectively (n=5), and that with isoprenaline 60.6+/-7.4% and 67.4+/-6.4%, respectively (n=5). These effects of NKH477 and isoprenaline on [Ca2+]i and tension were inhibited by 30 nM IbTX. The inhibitory action of IbTX was abolished in the presence of 1 microM nifedipine. 8. These results suggest that the bronchorelaxant action of NKH477 may result, at least in part, from activation of BK(Ca) channels, which may cause a hyperpolarization of smooth muscle cell membranes and a secondary decrease in Ca2+ influx through VDCs, leading to a decrease in [Ca2+]i.
Forskolin derivatives with increased selectivity for cardiac adenylyl cyclase.[Pubmed:9500868]
J Mol Cell Cardiol. 1998 Jan;30(1):97-108.
The current study was undertaken to examine whether we can target adenylyl cyclase to regulate beta-adrenergic signaling with increased cardiac selectivity. Forskolin, a natural diterpene compound, interacts directly with adenylyl cyclase. We studied the adenylyl cyclase isoform-selectivity of forskolin derivatives using insect cell membranes overexpressing type II, III, and V adenylyl cyclase isoforms. 6-[3-(dimethylamino) propionyl] forskolin (NKH477) stimulated type V more potently (1.87 +/- 0.02-fold) than type II (1.04 +/- 0.02-fold) and type III (0.89 +/- 0.03-fold) relative to forskolin (50 microM, P < 0.05). Similarly, 6-[3-(dimethylamino)propionyl]-14,15-dihydro-forskolin (DMAPD) stimulated type V (1.39 +/- 0.02-fold) more potently than types II (0.66 +/- 0.02-fold) and type III (0.31 +/- 0.02-fold) relative to forskolin (P < 0.05). This selectivity was maintained under different assay conditions--i.e. with different forskolin (0.1-100 microM) and Mg (1-10 mM) concentrations, with or without Gs alpha. NKH477 increased cAMP accumulation in HEK293 cells stably overexpressing type V more than forskolin (1.57 +/- 0.13-fold) (P < 0.05). Examination of multiple tissue homogenates revealed that DMAPD and NKH477 stimulated cardiac adenylyl cyclase more potently than the other tissue adenylyl cyclases (lung, brain, and kidney) relative to forskolin. Our results suggest that a particular side-chain modification of forskolin enhanced the selectivity for the cardiac isoform stimulation. Adenylyl cyclase isoforms may be targeted to increase tissue selectivity in future drug therapy for beta-adrenergic regulation.
Cardiovascular and adenylate cyclase stimulant properties of NKH477, a novel water-soluble forskolin derivative.[Pubmed:1380607]
J Cardiovasc Pharmacol. 1992 Apr;19(4):625-34.
The cardiovascular effects of NKH477 (6-(3-dimethylaminopropionyl)forskolin hydrochloride), a novel water-soluble forskolin derivative, were investigated in dogs. Intravenous (i.v.) injections of NKH477 (1-30 micrograms/kg) caused dose-related increases in left ventricular dP/dtmax (LVdP/dtmax), coronary and femoral artery blood flow (CBF, FBF), heart rate (HR), and myocardial oxygen consumption (MVO2) and a dose-related decrease in blood pressure (BP) in anesthetized dogs. The regression analysis between CBF and MVO2 showed that NKH477 did not influence substantially the balance of oxygen supply and demand. Infusions of NKH477 (0.15-0.6 microgram/kg/min i.v.) also increased LVdP/dtmax, cardiac output (CO), and HR and decreased BP, pulmonary arterial diastolic pressure, and total peripheral resistance (TPR) in a dose-dependent manner. In contrast to forskolin, NKH477 administered intraduodenally (0.05-0.2 mg/kg) and orally (0.15 and 0.3 mg/kg) clearly exhibited cardiovascular actions, as it did in i.v. administration, indicating that NKH477 is orally active. No arrhythmias were induced by NKH477 in any study. NKH477, like forskolin, showed adenylate cyclase stimulant activity in guinea pig ventricular membrane but did not inhibit Na+, K(+)-ATPase or phosphodiesterase (PDE) activity. Thus, NKH477 can be characterized as a potent, orally active, water-soluble forskolin derivative, which suggests that NKH477 is a useful inodilator for treatment of heart failure, especially in the severe stage with beta-adrenoceptor downregulation.


