Pomolic acidCAS# 13849-91-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13849-91-7 | SDF | Download SDF |
| PubChem ID | 382831 | Appearance | Powder |
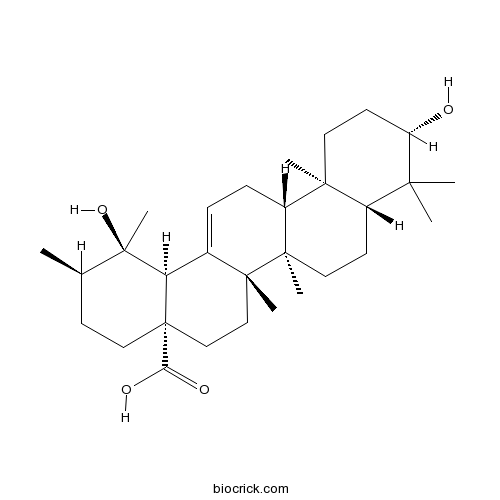
| Formula | C30H48O4 | M.Wt | 472.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2R,4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-1,10-dihydroxy-1,2,6a,6b,9,9,12a-heptamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid | ||
| SMILES | CC1CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)C)O)C)C)C2C1(C)O)C)C(=O)O | ||
| Standard InChIKey | ZZTYPLSBNNGEIS-OPAXANQDSA-N | ||
| Standard InChI | InChI=1S/C30H48O4/c1-18-10-15-30(24(32)33)17-16-27(5)19(23(30)29(18,7)34)8-9-21-26(4)13-12-22(31)25(2,3)20(26)11-14-28(21,27)6/h8,18,20-23,31,34H,9-17H2,1-7H3,(H,32,33)/t18-,20+,21-,22+,23-,26+,27-,28-,29-,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pomolic acid has anti-cancer, anti-inflammatory and apoptotic activities, it can induce apoptosis in SK-OV-3 cells, which is mediated by the mitochondrial-mediated intrinsic and death receptor-induced extrinsic pathways. Pomolic acid is a potent inhibitor of the aggregation of human platelets induced by ADP and Epinephrine, exhibits IC50 values close to 60 nM and 20 nM, respectively; pomolic acid does not inhibit human platelet aggregation induced by PAF, collagen, U46619 (thromboxane analogue), TRAP or arachidonic acid; suggests that the hypotensive and platelet anti-aggregating effects of pomolic acid and its potential role in cardiovascular therapy. |
| Targets | HIV | Caspase | AMPK | mTOR | NO |
| In vitro | Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids.[Pubmed: 9748372]J Nat Prod. 1998 Sep;61(9):1090-5.
Pomolic acid, triterpenoid isolated from Licania pittieri, as competitive antagonist of ADP-induced aggregation of human platelets.[Pubmed: 22402243]Phytomedicine. 2012 Apr 15;19(6):484-7.Pomolic acid (PA), triterpenoid isolated from Licania pittieri, has previously shown a potent ability to inhibit adenosine diphosphate (ADP)- and epinephrine-induced human platelet aggregation. |
| In vivo | Pomolic acid of Licania pittieri elicits endothelium-dependent relaxation in rat aortic rings.[Pubmed: 21112754]Phytomedicine. 2011 Apr 15;18(6):464-9.Pomolic acid has recently shown hypotensive effect in rats. The purpose of this investigation was to determine the vascular effects of this triterpenoid and to examine its mode of action. Anti-inflammatory and apoptotic activities of pomolic acid isolated from Cecropia pachystachya.[Pubmed: 18260049]Planta Med. 2008 Feb;74(3):215-20.
|
| Kinase Assay | Anticancer properties of pomolic acid-induced AMP-activated protein kinase activation in MCF7 human breast cancer cells.[Pubmed: 22223345]Biol Pharm Bull. 2012;35(1):105-10.AMP-activated protein kinase (AMPK) is a sensor of cellular energy status found in all eukaryotes. Recent studies indicate that AMPK activation strongly suppresses cell proliferation in tumor cells, which requires high rates of protein synthesis and de novo fatty acid synthesis for their rapid growth. Pomolic acid (PA) has been previously described as being active in inhibiting the growth of cancer cells. In this study, we investigated Pomolic acid activated AMPK, and this activity was related to proliferation and apoptosis in MCF7 breast cancer cells. Pomolic acid inhibited cell proliferation and induced sub-G(1) arrest, elevating the mRNA levels of the apoptotic genes p53 and p21. Pomolic acid activated caspase-3, -9, and poly(ADP-ribose) polymerase, and this effect was inhibited by z-VAD-fmk. AMPK activation was increased by treating cells with Pomolic acid, inactivated by treating cells with a compound C, and co-treatment consisting of Pomolic acid and aminoimidazole carboxamide ribonucleotide (AICAR) synergistically activated AMPK. These anti-cancer potentials of Pomolic acid were accompanied by effects on de novo fatty acid synthesis as shown by the decreased expression of fatty acid synthase, and decreased acetyl-CoA carboxylase activation and incorporation of [(3)H]acetyl-CoA into fatty acids. In addition, Pomolic acid inhibited key enzymes involved in protein synthesis such as mammalian target of rapamycin (mTOR), 70 kDa ribosomal protein S6 kinase (p70S6K), and eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1). |
| Cell Research | Pomolic acid induces apoptosis in SK-OV-3 human ovarian adenocarcinoma cells through the mitochondrial-mediated intrinsic and death receptor-induced extrinsic pathways.[Pubmed: 23255955 ]Oncol Lett. 2013 Jan;5(1):386-90.The cytotoxic effect of Pomolic acid (PA), a pentacyclic triterpene isolated from flowers of Osmanthus fragrans var. aurantiacus Makino, was investigated in SK-OV-3 human ovarian adenocarcinoma cells. |

Pomolic acid Dilution Calculator

Pomolic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1155 mL | 10.5775 mL | 21.1551 mL | 42.3101 mL | 52.8877 mL |
| 5 mM | 0.4231 mL | 2.1155 mL | 4.231 mL | 8.462 mL | 10.5775 mL |
| 10 mM | 0.2116 mL | 1.0578 mL | 2.1155 mL | 4.231 mL | 5.2888 mL |
| 50 mM | 0.0423 mL | 0.2116 mL | 0.4231 mL | 0.8462 mL | 1.0578 mL |
| 100 mM | 0.0212 mL | 0.1058 mL | 0.2116 mL | 0.4231 mL | 0.5289 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-Oxopomolic acid
Catalog No.:BCN3485
CAS No.:13849-90-6
- S-(+)-Marmesin
Catalog No.:BCN8288
CAS No.:13849-08-6
- Ro 31-8220 Mesylate
Catalog No.:BCC4999
CAS No.:138489-18-6
- FK 888
Catalog No.:BCC5907
CAS No.:138449-07-7
- A 1070722
Catalog No.:BCC7933
CAS No.:1384424-80-9
- Irbesartan
Catalog No.:BCC2560
CAS No.:138402-11-6
- Tinospinoside C
Catalog No.:BCN6925
CAS No.:1383977-51-2
- Afzelechin-(4alpha->8)-epiafzelechin
Catalog No.:BCN7709
CAS No.:1383627-30-2
- Boc-Arg(Tos)-OH
Catalog No.:BCC3067
CAS No.:13836-37-8
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- BD 1047 dihydrobromide
Catalog No.:BCC6863
CAS No.:138356-21-5
- BD 1008 dihydrobromide
Catalog No.:BCC6674
CAS No.:138356-09-9
- Tormentic acid
Catalog No.:BCN6198
CAS No.:13850-16-3
- 9-Hydroxycanthin-6-one
Catalog No.:BCN3105
CAS No.:138544-91-9
- N3PT
Catalog No.:BCC1782
CAS No.:13860-66-7
- NKH 477
Catalog No.:BCC7126
CAS No.:138605-00-2
- (R)-3-Carboxy-4-hydroxyphenylglycine
Catalog No.:BCC6606
CAS No.:13861-03-5
- Securitinine
Catalog No.:BCN6986
CAS No.:13861-71-7
- LP 20 hydrochloride
Catalog No.:BCC6266
CAS No.:1386928-34-2
- Eugenol rutinoside
Catalog No.:BCN6201
CAS No.:138772-01-7
- Fmoc-D-1-Nal-OH
Catalog No.:BCC3284
CAS No.:138774-49-3
- Fmoc-D-2-Nal-OH
Catalog No.:BCC3290
CAS No.:138774-94-4
- Fmoc-N-Me-Ile-OH
Catalog No.:BCC3214
CAS No.:138775-22-1
- Isomangiferolic acid
Catalog No.:BCN4768
CAS No.:13878-92-7
Anti-inflammatory and apoptotic activities of pomolic acid isolated from Cecropia pachystachya.[Pubmed:18260049]
Planta Med. 2008 Feb;74(3):215-20.
The dichloromethane extract and Pomolic acid ( 5) obtained from leaves of Cecropia pachystachya both reduced carrageenan-induced paw oedema in mice. Interestingly, while the triterpenoid inhibited the in vivo production of interleukin-1beta by 39 %, it had no effect on tumour necrosis factor-alpha production. We also demonstrated that both the dichloromethane extract and 5 inhibited the viability of human polymorphonuclear (PMN) cells in a time- and dose-dependent fashion. The PMN membrane integrity was determined with the aid of flow cytometry by means of the exclusion of propidium iodide as assay. Although the cell membrane integrity was altered, neither the extract nor 5 produced cellular necrosis. Moreover, the development of hypodiploid nuclei and DNA fragmentation in the PMN cells were both dependent on dose and time. Finally, in the annexin V-FITC binding assay, compound 5 increased the total of apoptotic cells by 42 % at 100 microM and by 71 % at 200 microM with respect to the control group. In conclusion, both the dichloromethane extract of ambay and isolated compound 5 inhibit the viability of PMN cells through apoptosis. Since they can regulate human neutrophil functions in this way, it is likely that these substances can also limit inflammation.
Pomolic acid, triterpenoid isolated from Licania pittieri, as competitive antagonist of ADP-induced aggregation of human platelets.[Pubmed:22402243]
Phytomedicine. 2012 Apr 15;19(6):484-7.
Pomolic acid (PA), triterpenoid isolated from Licania pittieri, has previously shown a potent ability to inhibit adenosine diphosphate (ADP)- and epinephrine-induced human platelet aggregation. To investigate whether PA could be an antagonist of ADP-activated receptors of human platelets (P2Y(1) and P2Y(12)), pharmacological studies were conducted to examining its ability to modulate the platelet shape change induced by a selective P2Y(1) receptor agonist MRS2365 and also the nature of its possible interaction with ADP receptors by analyzing the characteristics of log concentration-response curves of ADP constructed in the absence and in the presence of fixed concentrations of PA, using in vitro platelet aggregation assays. PA did not interfere with the activation of P2Y(1) receptor by MRS2365 to induce platelet shape change and displayed a competitive antagonism of ADP-induced platelet aggregation, which most probably involves competition for a single binding site in platelets. The estimated equilibrium dissociate constant (K(b)) of PA as ADP receptor antagonist was 15.4+/-0.06nM. Together, these findings give indirect evidence for the idea that PA could be a potent competitive antagonist of P2Y(12) receptor, and open the possibility to consider it as new member of the non-nucleotide generation of antiplatelet drugs.
Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids.[Pubmed:9748372]
J Nat Prod. 1998 Sep;61(9):1090-5.
Oleanolic acid (1) was identified as an anti-HIV principle from several plants, including Rosa woodsii (leaves), Prosopis glandulosa (leaves and twigs), Phoradendron juniperinum (whole plant), Syzygium claviflorum (leaves), Hyptis capitata (whole plant), and Ternstromia gymnanthera (aerial part). It inhibited HIV-1 replication in acutely infected H9 cells with an EC50 value of 1.7 microg/mL, and inhibited H9 cell growth with an IC50 value of 21.8 microg/mL [therapeutic index (T. I.) 12.8]. Pomolic acid, isolated from R. woodsii and H. capitata, was also identified as an anti-HIV agent (EC50 1.4 microg/mL, T. I. 16.6). Although ursolic acid did show anti-HIV activity (EC50 2.0 microg/mL), it was slightly toxic (IC50 6.5 microg/mL, T. I. 3.3). A new triterpene (11) was also isolated from the CHCl3-soluble fraction of R. woodsii, though it showed no anti-HIV activity. The structure of 11 was determined to be 1beta-hydroxy-2-oxoPomolic acid by spectral examination. Based on these results, we examined the anti-HIV activity of oleanolic acid- or Pomolic acid-related triterpenes isolated from several plants. In addition, we previously demonstrated that derivatives of betulinic acid, isolated from the leaves of S. claviflorum as an anti-HIV principle, exhibited extremely potent anti-HIV activity. Accordingly, we prepared derivatives of oleanolic acid and evaluated their anti-HIV activity. Among the oleanolic acid derivatives, 18 demonstrated most potent anti-HIV activity, with an EC50 value of 0. 0005 microg/mL and a T. I. value of 22 400.
Pomolic acid of Licania pittieri elicits endothelium-dependent relaxation in rat aortic rings.[Pubmed:21112754]
Phytomedicine. 2011 Apr 15;18(6):464-9.
Pomolic acid has recently shown hypotensive effect in rats. The purpose of this investigation was to determine the vascular effects of this triterpenoid and to examine its mode of action. Functional experiments in rat aortic rings precontracted with norepinephrine were performed to evaluate the vasorelaxant effect of Pomolic acid. This triterpenoid induced a vasorelaxation (IC(5)(0) = 2.45 muM) in a concentration- and endothelium-dependent manner and showed no effect on contractions evoked by KCl (25 mM). Pre-treatment of aortic rings with L-NAME (100 muM), methylene blue (100 muM) or glibenclamide (10 muM), totally prevented the vasorelaxation induced by Pomolic acid, while indomethacin (10 muM) had no effect on this response. Additionally, Pomolic acid relaxation was unaffected under the muscarinic- and beta-adrenergic-receptor blocked ensured for atropine and propanolol respectively (10 muM each). In contrast, the vasorelaxant effect of Pomolic acid was abolished under the purinergic-receptor blocked ensured for suramin (10 muM). Finally, apyrase (0.8 U/ml) an enzyme which hydrolyses ATP and ADP did not affect Pomolic acid relaxation. In summary, Pomolic acid has a potent endothelium-dependent vasorelaxant effect, possibly acting through the direct activation of endothelial purinergic receptors via NO-cGMP signaling pathway, which could be part of the mechanism underlying its hypotensive effect.
Pomolic acid induces apoptosis in SK-OV-3 human ovarian adenocarcinoma cells through the mitochondrial-mediated intrinsic and death receptor-induced extrinsic pathways.[Pubmed:23255955]
Oncol Lett. 2013 Jan;5(1):386-390.
The cytotoxic effect of Pomolic acid (PA), a pentacyclic triterpene isolated from flowers of Osmanthus fragrans var. aurantiacus Makino, was investigated in SK-OV-3 human ovarian adenocarcinoma cells. PA dose-dependently inhibited the viability of SK-OV-3 cells. PA-induced apoptosis was further characterized by detection of cell surface annexin V and sub-G1 apoptotic cell populations. The number of cells immunostained with annexin V-fluorescein isothiocyanate (FITC) increased following treatment with PA. The sub-G1 cell populations also increased in PA-treated SK-OV-3 cells. PA induced the activation of caspase-8, -9 and -3, critical mediators of apoptosis signaling. PA decreased the mitochondrial transmembrane potential (DeltaPsi(m)), resulting in the activation of caspase-9. In addition, PA increased the expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis signaling-related death receptor 5 (DR5), mediating caspase-8-involved extrinsic pathway. Taken together, our results indicate that PA induces apoptosis in SK-OV-3 cells, which is mediated by the mitochondrial-mediated intrinsic and death receptor-induced extrinsic pathways.
Anticancer properties of pomolic acid-induced AMP-activated protein kinase activation in MCF7 human breast cancer cells.[Pubmed:22223345]
Biol Pharm Bull. 2012;35(1):105-10.
AMP-activated protein kinase (AMPK) is a sensor of cellular energy status found in all eukaryotes. Recent studies indicate that AMPK activation strongly suppresses cell proliferation in tumor cells, which requires high rates of protein synthesis and de novo fatty acid synthesis for their rapid growth. Pomolic acid (PA) has been previously described as being active in inhibiting the growth of cancer cells. In this study, we investigated PA activated AMPK, and this activity was related to proliferation and apoptosis in MCF7 breast cancer cells. PA inhibited cell proliferation and induced sub-G(1) arrest, elevating the mRNA levels of the apoptotic genes p53 and p21. PA activated caspase-3, -9, and poly(ADP-ribose) polymerase, and this effect was inhibited by z-VAD-fmk. AMPK activation was increased by treating cells with PA, inactivated by treating cells with a compound C, and co-treatment consisting of PA and aminoimidazole carboxamide ribonucleotide (AICAR) synergistically activated AMPK. These anti-cancer potentials of PA were accompanied by effects on de novo fatty acid synthesis as shown by the decreased expression of fatty acid synthase, and decreased acetyl-CoA carboxylase activation and incorporation of [(3)H]acetyl-CoA into fatty acids. In addition, PA inhibited key enzymes involved in protein synthesis such as mammalian target of rapamycin (mTOR), 70 kDa ribosomal protein S6 kinase (p70S6K), and eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1). These results suggest that PA exerts anti-cancer properties through the modulation of AMPK pathways and its value as an anti-cancer agent in breast cancer therapy.


