Boc-Arg(Tos)-OHCAS# 13836-37-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13836-37-8 | SDF | Download SDF |
| PubChem ID | 294909 | Appearance | Powder |
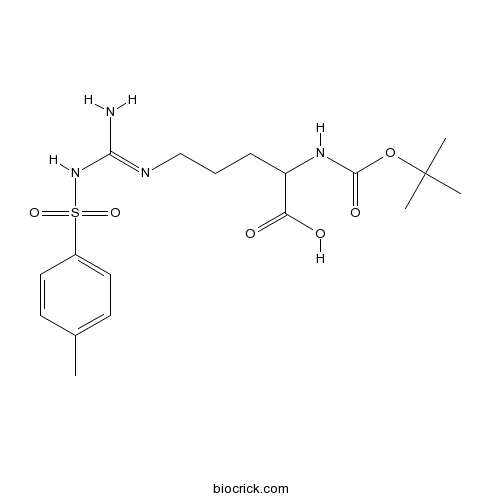
| Formula | C18H28N4O6S | M.Wt | 428.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water or 1% acetic acid | ||
| Chemical Name | 5-[[amino-[(4-methylphenyl)sulfonylamino]methylidene]amino]-2-[(2-methylpropan-2-yl)oxycarbonylamino]pentanoic acid | ||
| SMILES | CC1=CC=C(C=C1)S(=O)(=O)NC(=NCCCC(C(=O)O)NC(=O)OC(C)(C)C)N | ||
| Standard InChIKey | WBIIPXYJAMICNU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H28N4O6S/c1-12-7-9-13(10-8-12)29(26,27)22-16(19)20-11-5-6-14(15(23)24)21-17(25)28-18(2,3)4/h7-10,14H,5-6,11H2,1-4H3,(H,21,25)(H,23,24)(H3,19,20,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boc-Arg(Tos)-OH Dilution Calculator

Boc-Arg(Tos)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3337 mL | 11.6686 mL | 23.3372 mL | 46.6744 mL | 58.3431 mL |
| 5 mM | 0.4667 mL | 2.3337 mL | 4.6674 mL | 9.3349 mL | 11.6686 mL |
| 10 mM | 0.2334 mL | 1.1669 mL | 2.3337 mL | 4.6674 mL | 5.8343 mL |
| 50 mM | 0.0467 mL | 0.2334 mL | 0.4667 mL | 0.9335 mL | 1.1669 mL |
| 100 mM | 0.0233 mL | 0.1167 mL | 0.2334 mL | 0.4667 mL | 0.5834 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Boc-Arg(Tos)-OH
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- BD 1047 dihydrobromide
Catalog No.:BCC6863
CAS No.:138356-21-5
- BD 1008 dihydrobromide
Catalog No.:BCC6674
CAS No.:138356-09-9
- CYM 9484
Catalog No.:BCC6238
CAS No.:1383478-94-1
- Hedycoronen A
Catalog No.:BCN7653
CAS No.:1383441-73-3
- Stearyl glycyrrhetinate
Catalog No.:BCN8486
CAS No.:13832-70-7
- ML 289
Catalog No.:BCC6343
CAS No.:1382481-79-9
- (RS)-(Tetrazol-5-yl)glycine
Catalog No.:BCC6599
CAS No.:138199-51-6
- H-β-HoPhe-OH
Catalog No.:BCC3240
CAS No.:138165-77-2
- 7-Methoxy-1-naphthylacetonitrile
Catalog No.:BCN2242
CAS No.:138113-08-3
- Agomelatine
Catalog No.:BCN2165
CAS No.:138112-76-2
- (R,R)-THC
Catalog No.:BCC7224
CAS No.:138090-06-9
- Afzelechin-(4alpha->8)-epiafzelechin
Catalog No.:BCN7709
CAS No.:1383627-30-2
- Tinospinoside C
Catalog No.:BCN6925
CAS No.:1383977-51-2
- Irbesartan
Catalog No.:BCC2560
CAS No.:138402-11-6
- A 1070722
Catalog No.:BCC7933
CAS No.:1384424-80-9
- FK 888
Catalog No.:BCC5907
CAS No.:138449-07-7
- Ro 31-8220 Mesylate
Catalog No.:BCC4999
CAS No.:138489-18-6
- S-(+)-Marmesin
Catalog No.:BCN8288
CAS No.:13849-08-6
- 3-Oxopomolic acid
Catalog No.:BCN3485
CAS No.:13849-90-6
- Pomolic acid
Catalog No.:BCN6197
CAS No.:13849-91-7
- Tormentic acid
Catalog No.:BCN6198
CAS No.:13850-16-3
- 9-Hydroxycanthin-6-one
Catalog No.:BCN3105
CAS No.:138544-91-9
- N3PT
Catalog No.:BCC1782
CAS No.:13860-66-7
[Total solid phase synthesis of gamma-subunit of cGMP phosphodiesterase from bovine retina and physicochemical properties of synthetic protein].[Pubmed:9499369]
Bioorg Khim. 1997 Dec;23(12):933-48.
The 87-membered polypeptide with the sequence of the gamma subunit of cGMP phosphodiesterase from bovine retina rods (PDE gamma) was synthesized by the solid phase method. Two synthetic approaches, which were based on the Boc/Bzl-strategy, were used; both syntheses were carried out in a continuous-flow reactor with swellographic monitoring. In the first approach, five Arg residues were coupled in the form of Boc-Arg(Z)2-OH and the final cleavage of the peptide from the support was effected by the mixture of CF3SO2SiMe3 and thionisole in trifluoroacetic acid. There resulted a heterogeneous, ornitine-rich, and absolutely inactive peptide material which was insoluble in aqueous alkali. In the second approach, Arg(Tos) and the HF low-high cleavage procedure were used, which resulted in a homogeneous polypeptide (according to HPLC and capillary electrophoresis) that manifested correct molecular mass under ion-spray mass spectrometry and the full functional activity characteristic of the native protein. The effect of zinc salts on the PDE gamma fluorescence in solutions and on its solubility was established. This demonstrated a significant PDE gamma affinity with Zn2+ ions and appeared to be connected with the functioning of the protein in the retina cells. For the first time, the dynamics of the peptidylpolymer swelling in different solvents was studied during the synthesis of peptides with very long sequences.
New tris-alkoxycarbonyl arginine derivatives for peptide synthesis.[Pubmed:15635728]
J Pept Sci. 2005 Jan;11(1):60-4.
alpha-Alkoxycarbonyl protected ornithines were treated with N,N'-[Z(2Cl)](2)-S-methylisothiourea and N,N'-[Z(2Br)](2)-S-methylisothiourea, N,N'-Z(2)-S-methylisothiourea and N,N'-Boc(2)-S-methylisothiourea to form N(alpha, omega, omega')-tris-alkoxycarbonyl arginines. Two of them, Boc-Arg-{omega,omega'-[Z(2Br)](2)}-OH and Boc-Arg-{omega,omega'-[Z(2Cl)](2)}-OH, were used for the synthesis of dermorphin fragments containing two or three arginine residues. Examination of the products by HPLC and ESI-MS revealed that the purity of the materials obtained with the use of the new derivatives was higher than that obtained in concurrent syntheses in which Boc-Arg(Tos) was used.
Studies on lactam formation during coupling procedures of N alpha-N omega-protected arginine derivatives.[Pubmed:8738983]
Pept Res. 1996 Mar-Apr;9(2):88-91.
We evaluated the quantity of delta-lactam generated during the synthesis of arginine-containing dipeptides using Z-Arg(Tos)-OH, Boc-Arg(Tos)-OH, Fmoc-Arg(Boc)2-OH and Fmoc-Arg(Pmc)-OH and assayed several carboxyl-activating procedures for coupling the protected arginines to different amino components. We observed significant amounts of delta-lactam during the synthesis of Z-Arg(Tos)-methyl ester and Z-Arg(Tos)-amide, as well as of Boc-Arg(Tos)-chloromethyl ketone. The mixed anhydride coupling procedure and the di-Boc-protecting guanidino group induced more delta-lactam formation than any other coupling or NG-protection method. The amide, benzyl, 4-(NO2)-benzyl and methyl alpha-carboxyl-protected amino acids generated more delta-lactam than did those protected by tertbutyl or N2H2-Boc. So far it has not been possible to propose a general mechanism for delta-lactam formation or a process that completely abolishes it. Therefore, this side reaction should be considered almost inevitable. Its minimization requires examination of arginine-containing peptides in each specific synthesis.


