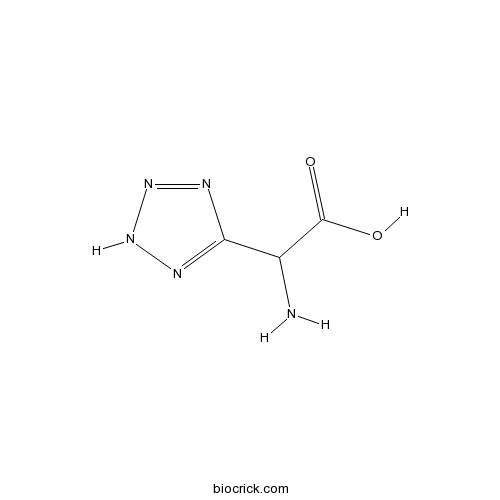
(RS)-(Tetrazol-5-yl)glycineCAS# 138199-51-6 |
- INCB3344
Catalog No.:BCC1648
CAS No.:1262238-11-8
- INCB8761(PF-4136309)
Catalog No.:BCC1649
CAS No.:1341224-83-6
- MK-0812
Catalog No.:BCC1755
CAS No.:624733-88-6
- INCB 3284 dimesylate
Catalog No.:BCC1646
CAS No.:887401-93-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 138199-51-6 | SDF | Download SDF |
| PubChem ID | 126383 | Appearance | Powder |
| Formula | C3H5N5O2 | M.Wt | 143.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in water | ||
| Chemical Name | 2-amino-2-(2H-tetrazol-5-yl)acetic acid | ||
| SMILES | C1(=NNN=N1)C(C(=O)O)N | ||
| Standard InChIKey | UKBRUIZWQZHXFL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C3H5N5O2/c4-1(3(9)10)2-5-7-8-6-2/h1H,4H2,(H,9,10)(H,5,6,7,8) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent NMDA receptor agonist, approximately 20 times more active than NMDA. Also available as part of the Mixed NMDA Receptor. |

(RS)-(Tetrazol-5-yl)glycine Dilution Calculator

(RS)-(Tetrazol-5-yl)glycine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.9881 mL | 34.9406 mL | 69.8812 mL | 139.7624 mL | 174.703 mL |
| 5 mM | 1.3976 mL | 6.9881 mL | 13.9762 mL | 27.9525 mL | 34.9406 mL |
| 10 mM | 0.6988 mL | 3.4941 mL | 6.9881 mL | 13.9762 mL | 17.4703 mL |
| 50 mM | 0.1398 mL | 0.6988 mL | 1.3976 mL | 2.7952 mL | 3.4941 mL |
| 100 mM | 0.0699 mL | 0.3494 mL | 0.6988 mL | 1.3976 mL | 1.747 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-β-HoPhe-OH
Catalog No.:BCC3240
CAS No.:138165-77-2
- 7-Methoxy-1-naphthylacetonitrile
Catalog No.:BCN2242
CAS No.:138113-08-3
- Agomelatine
Catalog No.:BCN2165
CAS No.:138112-76-2
- (R,R)-THC
Catalog No.:BCC7224
CAS No.:138090-06-9
- Acetylanonamine
Catalog No.:BCN2140
CAS No.:138079-62-6
- G007-LK
Catalog No.:BCC6383
CAS No.:1380672-07-0
- LDK378 dihydrochloride
Catalog No.:BCC1694
CAS No.:1380575-43-8
- 2-(4-Hydroxy-2-oxoindolin-3-yl)acetonitrile
Catalog No.:BCN1575
CAS No.:1380540-77-1
- YM 750
Catalog No.:BCC7542
CAS No.:138046-43-2
- EHop-016
Catalog No.:BCC5022
CAS No.:1380432-32-5
- KML 29
Catalog No.:BCC6312
CAS No.:1380424-42-9
- Valeriandoid B
Catalog No.:BCN6754
CAS No.:1380399-57-4
- ML 289
Catalog No.:BCC6343
CAS No.:1382481-79-9
- Stearyl glycyrrhetinate
Catalog No.:BCN8486
CAS No.:13832-70-7
- Hedycoronen A
Catalog No.:BCN7653
CAS No.:1383441-73-3
- CYM 9484
Catalog No.:BCC6238
CAS No.:1383478-94-1
- BD 1008 dihydrobromide
Catalog No.:BCC6674
CAS No.:138356-09-9
- BD 1047 dihydrobromide
Catalog No.:BCC6863
CAS No.:138356-21-5
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- Boc-Arg(Tos)-OH
Catalog No.:BCC3067
CAS No.:13836-37-8
- Afzelechin-(4alpha->8)-epiafzelechin
Catalog No.:BCN7709
CAS No.:1383627-30-2
- Tinospinoside C
Catalog No.:BCN6925
CAS No.:1383977-51-2
- Irbesartan
Catalog No.:BCC2560
CAS No.:138402-11-6
- A 1070722
Catalog No.:BCC7933
CAS No.:1384424-80-9
P2 purinoceptor blocker suramin antagonises NMDA receptors and protects against excitatory behaviour caused by NMDA receptor agonist (RS)-(tetrazol-5-yl)-glycine in rats.[Pubmed:9302084]
J Neurosci Res. 1997 Sep 1;49(5):627-38.
It has been reported that suramin, an anthelminthic, trypanocidal agent and an inhibitor of P2 receptors, may antagonise N-methyl-D-aspartate (NMDA) subtype of the excitatory amino acid receptors. Both NMDA receptors and P2X subclass of P2 receptors are ligand-gated Ca2+-selective channels and, since the increased influx of Ca2+ into neurons has been linked to neurotoxicity, simultaneous inhibition of P2X and NMDA receptors in vivo by suramin could represent an effective neuroprotective treatment. We have found that suramin inhibited the binding of [3H]CGP 39653 to NMDA receptor binding sites in vitro and reduced the frequency of NMDA channel openings in patch-clamp studies. Suramin (1 mM) had no effect on [3H]kainate binding in vitro. In vivo, intracerebroventricular (I.C.V.) injections of suramin (70 nmol/brain) antagonised convulsive effects of the NMDA agonist (RS)-(tetrazol-5-yl)-glycine (TZG, LY 285265). Suramin, however, did not prevent neurotoxic lesions in the hippocampus caused by I.C.V. administration of TZG. Increasing the dose of suramin resulted in death from severe respiratory depression.
Differentiation of in vivo effects of AMPA and NMDA receptor ligands using drug discrimination methods and convulsant/anticonvulsant activity.[Pubmed:8575516]
Eur J Pharmacol. 1995 Oct 24;285(3):289-97.
The discriminative stimulus properties of the AMPA ((RS)-2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionic acid) receptor agonist ATPA ((RS)-2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl)propionic acid) and NMDA (N-methyl-D-aspartic acid) in rats have been characterized. It is suggested that the cues are mediated by separate mechanisms in the central nervous system. The ATPA cue is not mimicked by NMDA or an NMDA receptor agonist, and is inhibited by the AMPA receptor antagonist (R)-APPA ((R)-2-amino-3-(3-hydroxy-5-phenylisoxazol-4-yl)propionic acid) but not the AMPA receptor antagonist ATOA ((RS)-2-amino-3-(3-carboxymethoxy-5-tert-butylisoxazol-4-yl)propio nic acid) or the NMDA receptor antagonist CPP ((RS)-3-(2-carboxypiperazin-4-yl)propyl)phosphonic acid). The ATPA cue is not mimicked by AMPA which is believed not to penetrate the blood-brain barrier. In contrast, ATPA does not generalize to the NMDA cue, which is mimicked by some NMDA receptor agonists (tetrazol-5-yl-glycine and AMAA ((RS)-2-amino-2-(3-hydroxy-5-methylisoxazol-4-yl)acetic acid)) and is inhibited by the NMDA receptor antagonist CPP. Highly potent convulsant activity was demonstrated in mice with all AMPA and NMDA receptor agonists after intracerebroventricular (i.c.v.) injection, whereas weaker or no effects were found after subcutaneous (s.c.) or intravenous injection. Only (RS)-tetrazol-5-yl-glycine had a potent effect after s.c. administration. I.c.v. ATOA and CPP inhibited convulsions induced by i.c.v. injection of AMPA or NMDA, while (R)-APPA was ineffective. These results indicate that there are differences in the structure-activity relations in the drug discrimination and convulsant/anticonvulsant models, even when effects after i.c.v. and s.c. injection are taken into consideration. The convulsion models are rapid tests which can give an indication of central nervous system penetration, but are less pharmacologically specific with respect to differentiation between AMPA and NMDA ligands where cue models demonstrate clear differences in effects of ligands with selectivity for receptor subtypes.
NMDA receptor heterogeneity in mammalian tissues: focus on two agonists, (2S,3R,4S) cyclopropylglutamate and the sulfate ester of 4-hydroxy-(S)-pipecolic acid.[Pubmed:7543185]
Naunyn Schmiedebergs Arch Pharmacol. 1995 Apr;351(4):371-6.
Several potent and selective agonists of the glutamate (L-GLU) receptors of N-methyl-D-aspartate (NMDA) type have been tested on the L-[3H]GLU binding to rat cortical membranes, on the depolarization of mouse cortical wedges and on the contraction of guinea pig longitudinal muscle myenteric plexus preparations with the aim of comparing the NMDA receptors present in the cortex and those present in the gut. When the depolarization of the cortical wedges was evaluated, the EC50 values of the agonists were (microM): (R,S)-(tetrazol-5-yl)-glycine (TG) 0.3; trans-4-hydroxy-(S)-pipecolic acid-4-sulfate (t-HPIS) 0.7; 1-aminocyclobutane-cis-1,3-dicarboxylic acid (ACBD) 0.8; NMDA 8; (2S,3R,4S) cyclopropylglutamate (L-CGA C) 12; quinolinic acid (QUIN) 400. When the contraction of the longitudinal muscle myenteric plexus was evaluated, the EC50 values were (microM): L-CGA C 1; TG 8; ACBD 50; t-HPIS 100; QUIN 500 and NMDA 680. When the displacement of NMDA specific L-[3H]GLU binding from rat cortical membranes was evaluated, the IC50 values were (microM): L-CGA C 0.003; TG 0.005; ACBD 0.044; t-HPIS 0.062; NMDA 0.31 and QUIN 15. No significant correlation was found when the EC50 values obtained in the ileum were plotted against the EC50 values obtained in the cortex (r = 0.47). In particular it was noted that L-CGA C was approximately three orders of magnitude more potent than NMDA when tested in the ileum but had a potency not significantly different from that of NMDA when tested in the cortex.(ABSTRACT TRUNCATED AT 250 WORDS)
D,L-(tetrazol-5-yl) glycine: a novel and highly potent NMDA receptor agonist.[Pubmed:1686860]
Eur J Pharmacol. 1991 Oct 15;203(2):237-43.
This paper describes the pharmacological activity of D,L-(tetrazol-5-yl)glycine, a structurally novel and highly potent agonist at the N-methyl-D-aspartate (NMDA) subtype of excitatory amino acid receptor. D,L-(Tetrazol-5-yl)glycine potently displaced NMDA receptor binding to rat brain membranes as measured using [3H]CGS19755 (IC50 = 98 +/- 7 nM) and [3H]glutamate (IC50 = 36 +/- 18 nM) as ligands. D,L-(Tetrazol-5-yl)glycine did not appreciably inhibit the binding of D,L-alpha-[5-methyl-3H] amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA), [3H]kainate, or [3H]glycine (IC50s greater than 30,000 nM). D,L-(Tetrazol-5-yl)glycine was more potent than NMDA or cis-methanoglutamate as a depolarizing agent in the rat cortical slice, and unlike these other agents induced rapid receptor-mediated neurotoxicity. Depolarization by D,L-(tetrazol-5-yl)glycine was antagonized by LY233053, a selective NMDA receptor antagonist. D,L-(Tetrazol-5-yl)glycine was a highly potent convulsant when given to neonatal rats (ED50 = 0.071 mg/kg i.p.). Convulsions in neonatal rats or lethality in mice induced by D,L-(tetrazol-5-yl)glycine were selectively antagonized by competitive and non-competitive NMDA receptor antagonists. D,L-(Tetrazol-5-yl)glycine is a structurally novel (tetrazole-substituted) compound that is a highly potent and selective NMDA receptor agonist. D,L-(Tetrazol-5-yl)glycine could be used to probe further NMDA receptor function in vitro and in vivo.


