KML 29Highly potent and selective MAGL inhibitor CAS# 1380424-42-9 |
- Vatalanib (PTK787) 2HCl
Catalog No.:BCC1111
CAS No.:212141-51-0
- Cediranib (AZD217)
Catalog No.:BCC1121
CAS No.:288383-20-0
- Lenvatinib (E7080)
Catalog No.:BCC1172
CAS No.:417716-92-8
- Tivozanib (AV-951)
Catalog No.:BCC1179
CAS No.:475108-18-0
- Brivanib (BMS-540215)
Catalog No.:BCC1231
CAS No.:649735-46-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1380424-42-9 | SDF | Download SDF |
| PubChem ID | 71656212 | Appearance | Powder |
| Formula | C24H21F6NO7 | M.Wt | 549.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (91.01 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
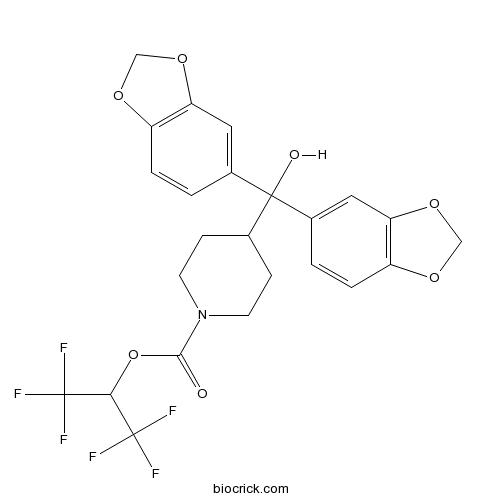
| Chemical Name | 1,1,1,3,3,3-hexafluoropropan-2-yl 4-[bis(1,3-benzodioxol-5-yl)-hydroxymethyl]piperidine-1-carboxylate | ||
| SMILES | C1CN(CCC1C(C2=CC3=C(C=C2)OCO3)(C4=CC5=C(C=C4)OCO5)O)C(=O)OC(C(F)(F)F)C(F)(F)F | ||
| Standard InChIKey | SXHQLPHDBLTFPM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H21F6NO7/c25-23(26,27)20(24(28,29)30)38-21(32)31-7-5-13(6-8-31)22(33,14-1-3-16-18(9-14)36-11-34-16)15-2-4-17-19(10-15)37-12-35-17/h1-4,9-10,13,20,33H,5-8,11-12H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly selective and potent monoacylglycerol lipase (MAGL) inhibitor. Exhibits potent inhibition of human, mouse and rat MAGL (IC50 values are 5.9, 15 and 43 nM, respectively). Exhibits no detectable inhibition of FAAH (IC50 > 50000 nM). Potently and selectively blocks hydrolysis of 2-arachidonoylglycerol (2-AG) in mice (IC50 = 2.5 nM and >50 μM for 2-AG and AEA respectively). |

KML 29 Dilution Calculator

KML 29 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8201 mL | 9.1005 mL | 18.201 mL | 36.402 mL | 45.5025 mL |
| 5 mM | 0.364 mL | 1.8201 mL | 3.6402 mL | 7.2804 mL | 9.1005 mL |
| 10 mM | 0.182 mL | 0.9101 mL | 1.8201 mL | 3.6402 mL | 4.5503 mL |
| 50 mM | 0.0364 mL | 0.182 mL | 0.364 mL | 0.728 mL | 0.9101 mL |
| 100 mM | 0.0182 mL | 0.091 mL | 0.182 mL | 0.364 mL | 0.455 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
KML29 is a potent and selective MAGL inhibitor with IC50 = 5.9, 15, and 43 nM in human, mouse, and rat brain proteomes, respectively. IC50 value: 15, 43, and 5.9 nM (mouse, rat, and human brain proteomes) Target: MAGL in vitro: KML29 potently and selectively inhibits MAGL with minimal cross-reactivity toward other central and peripheral serine hydrolases, including no detectable activity against FAAH.[1] in vivo: KML29 a potentially very useful tool to explore the consequences of inhibiting MAGL in the whole animal and in multiple species, and provides greater selectivity than JZL184 in inhibiting MAGL. [2]
References:
[1]. Chang JW, et al. Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem Biol. 2012 May 25;19(5):579-588.
[2]. Ignatowska-Jankowska BM,et al. In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: antinociceptive activity without cannabimimetic side effects. Br J Pharmacol. 2014 Mar;171(6):1392-1407.
- Valeriandoid B
Catalog No.:BCN6754
CAS No.:1380399-57-4
- EPZ004777 HCl
Catalog No.:BCC4550
CAS No.:1380316-03-9
- EPZ5676
Catalog No.:BCC2215
CAS No.:1380288-87-8
- KB SRC 4
Catalog No.:BCC6253
CAS No.:1380088-03-8
- BET bromodomain inhibitor
Catalog No.:BCC6426
CAS No.:1380087-89-7
- Decorticasine
Catalog No.:BCN2006
CAS No.:1380-03-6
- Limonene
Catalog No.:BCN3797
CAS No.:138-86-3
- Shikimic acid
Catalog No.:BCN6200
CAS No.:138-59-0
- Picrocrocine
Catalog No.:BCC8232
CAS No.:138-55-6
- D-(-)-Salicin
Catalog No.:BCN6298
CAS No.:138-52-3
- Mafenide
Catalog No.:BCC5237
CAS No.:138-39-6
- Atroscine
Catalog No.:BCN1941
CAS No.:138-12-5
- EHop-016
Catalog No.:BCC5022
CAS No.:1380432-32-5
- YM 750
Catalog No.:BCC7542
CAS No.:138046-43-2
- 2-(4-Hydroxy-2-oxoindolin-3-yl)acetonitrile
Catalog No.:BCN1575
CAS No.:1380540-77-1
- LDK378 dihydrochloride
Catalog No.:BCC1694
CAS No.:1380575-43-8
- G007-LK
Catalog No.:BCC6383
CAS No.:1380672-07-0
- Acetylanonamine
Catalog No.:BCN2140
CAS No.:138079-62-6
- (R,R)-THC
Catalog No.:BCC7224
CAS No.:138090-06-9
- Agomelatine
Catalog No.:BCN2165
CAS No.:138112-76-2
- 7-Methoxy-1-naphthylacetonitrile
Catalog No.:BCN2242
CAS No.:138113-08-3
- H-β-HoPhe-OH
Catalog No.:BCC3240
CAS No.:138165-77-2
- (RS)-(Tetrazol-5-yl)glycine
Catalog No.:BCC6599
CAS No.:138199-51-6
- ML 289
Catalog No.:BCC6343
CAS No.:1382481-79-9
Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates.[Pubmed:22542104]
Chem Biol. 2012 May 25;19(5):579-88.
The endocannabinoids 2-arachidonoyl glycerol (2-AG) and N-arachidonoyl ethanolamine (anandamide) are principally degraded by monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH), respectively. The recent discovery of O-aryl carbamates such as JZL184 as selective MAGL inhibitors has enabled functional investigation of 2-AG signaling pathways in vivo. Nonetheless, JZL184 and other reported MAGL inhibitors still display low-level cross-reactivity with FAAH and peripheral carboxylesterases, which can complicate their use in certain biological studies. Here, we report a distinct class of O-hexafluoroisopropyl (HFIP) carbamates that inhibits MAGL in vitro and in vivo with excellent potency and greatly improved selectivity, including showing no detectable cross-reactivity with FAAH. These findings designate HFIP carbamates as a versatile chemotype for inhibiting MAGL and should encourage the pursuit of other serine hydrolase inhibitors that bear reactive groups resembling the structures of natural substrates.


