NaringinCAS# 10236-47-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10236-47-2 | SDF | Download SDF |
| PubChem ID | 442428 | Appearance | Beige powder |
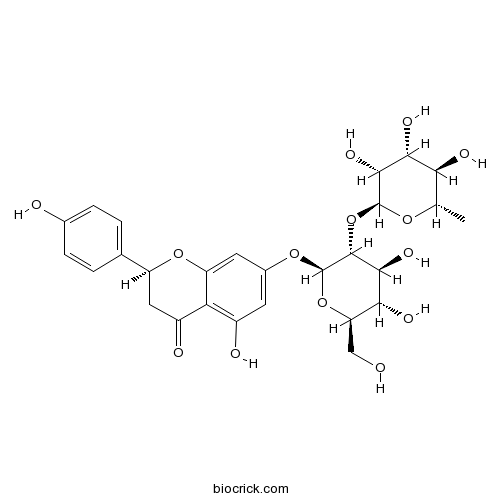
| Formula | C27H32O14 | M.Wt | 580.53 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Naringoside | ||
| Solubility | DMSO : 1 mg/mL (1.72 mM; Need ultrasonic) H2O : 1 mg/mL (1.72 mM; ultrasonic and warming and heat to 80°C) | ||
| Chemical Name | (2S)-7-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-5-hydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one | ||
| SMILES | CC1C(C(C(C(O1)OC2C(C(C(OC2OC3=CC(=C4C(=O)CC(OC4=C3)C5=CC=C(C=C5)O)O)CO)O)O)O)O)O | ||
| Standard InChIKey | DFPMSGMNTNDNHN-ZPHOTFPESA-N | ||
| Standard InChI | InChI=1S/C27H32O14/c1-10-20(32)22(34)24(36)26(37-10)41-25-23(35)21(33)18(9-28)40-27(25)38-13-6-14(30)19-15(31)8-16(39-17(19)7-13)11-2-4-12(29)5-3-11/h2-7,10,16,18,20-30,32-36H,8-9H2,1H3/t10-,16-,18+,20-,21+,22+,23-,24+,25+,26-,27+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Naringin exhibits antioxidant, anti-atherogenic, antiulcer, anti-hypocholesterolemic, anti-lipoperoxidative, and anti-hyperglycemia effects. Naringin reduces Ara-C-induced oxidative stress through both an inhibition of the generation of ROS production and an increase in antioxidant enzyme activities, it blocks apoptosis caused by Ara-C-induced oxidative stress, resulting in the inhibition of the cytotoxicity of Ara-C. Naringin attenuates epidermal growth factor (EGF)-induced MUC5AC secretion in A549 cells by suppressing the cooperative activities of MAPKs-AP-1 and IKKs-IκB-NF-κB signaling pathways. |
| Targets | p21 | Wnt/β-catenin | p38MAPK | JNK | NF-kB | p65 | AP-1 | IkB | EGFR | IKK |
| In vitro | Effects of naringin on hydrogen peroxide-induced cytotoxicity and apoptosis in P388 cells.[Pubmed: 12832847]J Pharmacol Sci. 2003 Jun;92(2):166-70.Flavonoids are widely recognized as naturally occurring antioxidants. Naringin (NG) is one of the flavonoid components in citrus fruits such as grapefruit. Hydrogen peroxide (H2O2) causes cytotoxicity through oxidative stress and apoptosis. |
| In vivo | Effect of naringin on hemodynamic changes and left ventricular function in renal artery occluded renovascular hypertension in rats.[Pubmed: 25883516]J Pharm Bioallied Sci. 2015 Apr-Jun;7(2):121-7.Renal artery occlusion (RAO) induced hypertension is a major health problem associated with structural and functional variations of the renal and cardiac vasculature. Naringin a flavanone glycoside derived possesses metal-chelating, antioxidant and free radical scavenging properties. The objective of this study was to investigate the antihypertensive activity of Naringin in RAO induced hypertension in rats. Nitric oxide mechanism in the protective effect of naringin against post-stroke depression (PSD) in mice.[Pubmed: 20433854 ]Life Sci. 2010 Jun 19;86(25-26):928-35.The present study has been designed to explore the nitric oxide mechanism in the protective effect of Naringin against I/R induced neurobehavioral alterations, oxidative damage and mitochondrial dysfunction in mice.
Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats.[Pubmed: 17188415 ]Toxicology. 2007 Feb 12;230(2-3):178-88.Diets rich in natural antioxidants are associated with reduced risk of heart diseases. Antiulcer effect of naringin on gastric lesions induced by ethanol in rats.[Pubmed: 7972328]Pharmacology. 1994 Sep;49(3):144-50.This study was designed to determine the gastroprotective properties of Naringin on and the involvement of endogenous prostaglandins in mucosal injury produced by absolute ethanol. |
| Kinase Assay | Naringin attenuates EGF-induced MUC5AC secretion in A549 cells by suppressing the cooperative activities of MAPKs-AP-1 and IKKs-IκB-NF-κB signaling pathways.[Pubmed: 22766066 ]Naringin inhibits growth potential of human triple-negative breast cancer cells by targeting β-catenin signaling pathway.[Pubmed: 23694763 ]Toxicol Lett. 2013 Jul 18;220(3):219-28.Triple-negative (ER-/PR-/HER2-) breast cancer (TNBC) is a severe clinical problem because of its relatively poorer prognosis, aggressive behavior and lack of targeted therapies. Naringin, a major flavonoid extracted from citrus fruits, has been reported to exert promising anticancer activities. However, the detailed antitumor mechanism of Naringin still remains enigmatic. Eur J Pharmacol. 2012 Sep 5;690(1-3):207-13.Naringenin, the aglycone of Naringin, has been reported to attenuate MUC5AC secretion by inhibiting activity of nuclear factor kappa B (NF-κB) via EGFR-PI3K-Akt/ERK MAPKinase signaling pathways. However, previous studies demonstrated that the MUC5AC promoter was located in two different regions: an activator protein-1 (AP-1) binding site and a NF-κB binding site. |
| Animal Research | Protective effect of naringin, a citrus flavonoid, against colchicine-induced cognitive dysfunction and oxidative damage in rats.[Pubmed: 20673063 ]Antihypercholesterolemic property of naringin alters plasma and tissue lipids, cholesterol-regulating enzymes, fecal sterol and tissue morphology in rabbits.[Pubmed: 15380892 ]The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice.[Pubmed: 15465737]J. Nutr., 2004, 134(10):2499-503.Dietary antioxidant compounds such as bioflavonoids may offer some protection against the early stage of diabetes mellitus and the development of complications. Clin Nutr. 2004 Oct;23(5):1025-34.Hyperlipidemia is a major risk factor for cardiovascular diseases. This study was designed to confirm the hypocholesterolemic role of Naringin.
J Med Food. 2010 Aug;13(4):976-84.Alzheimer's disease is a neurodegenerative disorder. Central administration of colchicine is well known to cause cognitive impairment and oxidative damage, which simulates sporadic dementia of the Alzheimer type in humans. |

Naringin Dilution Calculator

Naringin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7226 mL | 8.6128 mL | 17.2256 mL | 34.4513 mL | 43.0641 mL |
| 5 mM | 0.3445 mL | 1.7226 mL | 3.4451 mL | 6.8903 mL | 8.6128 mL |
| 10 mM | 0.1723 mL | 0.8613 mL | 1.7226 mL | 3.4451 mL | 4.3064 mL |
| 50 mM | 0.0345 mL | 0.1723 mL | 0.3445 mL | 0.689 mL | 0.8613 mL |
| 100 mM | 0.0172 mL | 0.0861 mL | 0.1723 mL | 0.3445 mL | 0.4306 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Naringin is a major flavanone glycoside obtained from tomatoes, grapefruits, and many other citrus fruits. Naringin exhibits biological properties such as antioxidant, anti-inflammatory, and antiapoptotic activities.
In Vitro:Naringin suppresses NF-κ B signaling pathway activation. Naringenin inhibits high glucose-induced proliferation, inflammatory reaction and oxidative stress injury in HBZY-1 cells[1]. Naringin inhibits AGS cancer cell proliferation in a dose- and time-dependent manner. Phosphorylation of PI3K and its activated downstream targets p-Akt and p-mTOR are significantly decreased at 2 mM in Naringin-treated AGS cells. Naringin induces autophagic cell death in AGS cells. Naringin activated the autophagy related protein in AGS cells[2]. Naringin protects PC12 cells from 3-NP neurotoxicity. The lactate dehydrogenase release is decreased upon naringin treatment in 3-NP-induced PC12 cells. Naringin treatment enhances the antioxidant defense by increasing the activities of enzymatic antioxidants and the level of reduced glutathione[3].
In Vivo:Treatment with naringin significantly alleviates renal injury in diabetic rats and increases diabetic rats body weight significantly. Administration of naringin effectively alleviates the collagen deposition and renal interstitial fibrosis in diabetic rats. Treatment with naringin could result in decreased levels of ROS and MDA and increased activities of SOD and GSH-Px[1]. Oral administration of naringin significantly improves the learning and memory abilities. Naringin significantly enhances insulin signaling pathway[3].
References:
[1]. Chen F, et al. Naringin Alleviates Diabetic Kidney Disease through Inhibiting Oxidative Stress and Inflammatory Reaction. PLoS One. 2015 Nov 30;10(11):e0143868.
[2]. Raha S, et al. Naringin induces autophagy-mediated growth inhibition by downregulating the PI3K/Akt/mTOR cascade via activation of MAPK pathways in AGS cancer cells. Int J Oncol. 2015 Sep;47(3):1061-9.
[3]. Kulasekaran G, et al. Neuroprotective efficacy of naringin on 3-nitropropionic acid-induced mitochondrial dysfunction through the modulation of Nrf2 signaling pathway in PC12 cells. Mol Cell Biochem. 2015 Nov;409(1-2):199-211.
[4]. Wang D, et al. Naringin Improves Neuronal Insulin Signaling, Brain Mitochondrial Function, and Cognitive Function in High-Fat Diet-Induced Obese Mice. Cell Mol Neurobiol. 2015 Oct;35(7):1061-71.
- Cadensin D
Catalog No.:BCN7260
CAS No.:102349-35-9
- (R)-Eriodictyol-8-C-beta-D-glucopyranoside
Catalog No.:BCN8028
CAS No.:1023271-51-3
- Epoxyparvinolide
Catalog No.:BCN5838
CAS No.:102227-61-2
- SGX-523
Catalog No.:BCC1055
CAS No.:1022150-57-7
- Btk inhibitor 1 R enantiomer
Catalog No.:BCC5125
CAS No.:1022150-12-4
- ARN2966
Catalog No.:BCC8074
CAS No.:102212-26-0
- Tyrosine kinase inhibitor
Catalog No.:BCC2020
CAS No.:1021950-26-4
- Boc-4-oxo-Pro-OMe
Catalog No.:BCC3436
CAS No.:102195-80-2
- 3-O-Methyltirotundin
Catalog No.:BCN5837
CAS No.:1021945-29-8
- ZM 39923 HCl
Catalog No.:BCC2203
CAS No.:1021868-92-7
- AGN 192403 hydrochloride
Catalog No.:BCC6924
CAS No.:1021868-90-5
- ARL 67156 trisodium salt
Catalog No.:BCC7004
CAS No.:1021868-83-6
- Negsehisandrin G
Catalog No.:BCN2674
CAS No.:1023744-69-5
- Glyburide
Catalog No.:BCC4784
CAS No.:10238-21-8
- AF-DX 116
Catalog No.:BCC6939
CAS No.:102394-31-0
- Jasplakinolide
Catalog No.:BCC7485
CAS No.:102396-24-7
- Fmoc-Phg-OH
Catalog No.:BCC3312
CAS No.:102410-65-1
- RN 1747
Catalog No.:BCC7769
CAS No.:1024448-59-6
- Kushenol N
Catalog No.:BCN2984
CAS No.:102490-65-3
- GK921
Catalog No.:BCC8057
CAS No.:1025015-40-0
- Periglaucine A
Catalog No.:BCN5839
CAS No.:1025023-04-4
- Periglaucine B
Catalog No.:BCN7053
CAS No.:1025023-05-5
- SGI-1776 free base
Catalog No.:BCC2232
CAS No.:1025065-69-3
- (-)-Huperzine A
Catalog No.:BCN1057
CAS No.:102518-79-6
Effect of naringin on hemodynamic changes and left ventricular function in renal artery occluded renovascular hypertension in rats.[Pubmed:25883516]
J Pharm Bioallied Sci. 2015 Apr-Jun;7(2):121-7.
BACKGROUND: Renal artery occlusion (RAO) induced hypertension is a major health problem associated with structural and functional variations of the renal and cardiac vasculature. Naringin a flavanone glycoside derived possesses metal-chelating, antioxidant and free radical scavenging properties. OBJECTIVE: The objective of this study was to investigate the antihypertensive activity of Naringin in RAO induced hypertension in rats. MATERIAL AND METHODS: Male Wistar rats (180-200 g) were divided into five groups Sham, RAO, Naringin (20, 40 and 80 mg/kg). Animals were pretreated with Naringin (20, 40 and 80 mg/kg p.o) for 4 weeks. On the last day of the experiment, left renal artery was occluded with renal bulldog clamp for 4 h. After assessment of hemodynamic and left ventricular function various biochemical (superoxide dismutase [SOD], glutathione [GSH] and malondialdehyde [MDA]) and histological parameters were determined in the kidney. RESULTS: RAO group significantly (P < 0.001) increased hemodynamic parameters at 15, 30 and 45 min of clamp removal. Naringin (40 and 80 mg/kg) treated groups showed a significant decrease in hemodynamic parameters at 15 min. after clamp removal that remained sustained for 60 min. Naringin (40 and 80 mg/kg) treated groups showed significant improvement in left ventricular function at 15, 30 and 45 min after clamp removal. Alteration in level of SOD, GSH and MDA was significantly restored by Naringin (40 and 80 mg/kg) treatment. It also reduced histological aberration induced in kidney by RAO. CONCLUSION: It is concluded that the antihypertensive activity of Naringin may result through inhibition of oxidative stress.
The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice.[Pubmed:15465737]
J Nutr. 2004 Oct;134(10):2499-503.
Dietary antioxidant compounds such as bioflavonoids may offer some protection against the early stage of diabetes mellitus and the development of complications. We investigated the effect of citrus bioflavonoids on blood glucose level, hepatic glucose-regulating enzymes activities, hepatic glycogen concentration, and plasma insulin levels, and assessed the relations between plasma leptin and body weight, blood glucose, and plasma insulin. Male C57BL/KsJ-db/db mice (db/db mice, 5 wk old), an animal model for type 2 diabetes, were fed a nonpurified diet for 2 wk and then were fed an AIN-76 control diet or the control diet supplemented with hesperidin (0.2 g/kg diet) or Naringin (0.2 g/kg diet). Hesperidin and Naringin supplementation significantly reduced blood glucose compared with the control group. Hepatic glucokinase activity and glycogen concentration were both significantly elevated in the hesperidin- and the Naringin-supplemented groups compared with the control group. Naringin also markedly lowered the activity of hepatic glucose-6-phosphatase and phosphoenolpyruvate carboxykinase compared with the control group. Plasma insulin, C-peptide, and leptin levels in the db/db mice from the 2 bioflavonoid-supplemented groups were significantly higher than those of the control group. Furthermore, plasma leptin was positively correlated with plasma insulin level (r = 0.578, P < 0.01) and body weight (r = 0.541, P < 0.05), and was inversely correlated with the blood glucose level (r = -0.46, P < 0.05). The current results suggest that hesperidin and Naringin both play important roles in preventing the progression of hyperglycemia, partly by increasing hepatic glycolysis and glycogen concentration and/or by lowering hepatic gluconeogenesis.
Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats.[Pubmed:17188415]
Toxicology. 2007 Feb 12;230(2-3):178-88.
Diets rich in natural antioxidants are associated with reduced risk of heart diseases. This study was aimed to evaluate the preventive role of Naringin on cardiac troponin T (cTnT), lactate dehydrogenase (LDH)-isoenzyme, cardiac marker enzymes, electrocardiographic (ECG)-patterns and lysosomal enzymes in isoproterenol (ISO)-induced myocardial infarction (MI) in male Wistar rats. Rats subcutaneously injected with ISO (85mg/kg) at an interval of 24h for 2 days showed a significant increase in the levels of cTnT, intensity of the bands of LDH-isoenzyme (LDH1 and LDH2) and the activities of cardiac marker enzymes such as creatine kinase-MB (CK-MB), creatine kinase (CK), LDH, aspartate transaminase (AST) and alanine transaminase (ALT) in serum with subsequent decrease in the activities of CK, LDH, AST and ALT in the heart and alterations in ECG-patterns. The activities of lysosomal enzymes (beta-glucuronidase, beta-N-acetyl glucosaminidase, beta-galactosidase, cathepsin-B and cathepsin-D) were increased significantly in serum and the heart of ISO-induced rats, but the activities of beta-glucuronidase and cathepsin-D were decreased significantly in the lysosomal fraction of the heart. Pretreatment with Naringin (10, 20 or 40mg/kg) daily for a period of 56 days positively altered the levels of cTnT, intensity of the bands of the LDH1 and LDH2-isoenzyme and the activities of cardiac marker enzymes, ECG-patterns and lysosomal hydrolases in ISO-induced rats. Thus, Naringin possess cardioprotective effect in ISO-induced MI in rats.
Protective effect of naringin, a citrus flavonoid, against colchicine-induced cognitive dysfunction and oxidative damage in rats.[Pubmed:20673063]
J Med Food. 2010 Aug;13(4):976-84.
Alzheimer's disease is a neurodegenerative disorder. Central administration of colchicine is well known to cause cognitive impairment and oxidative damage, which simulates sporadic dementia of the Alzheimer type in humans. The present study has been designed to investigate the protective effects of Naringin against the colchicine-induced cognitive impairment and oxidative damage in rats. Colchicine (15 microg/5 microL), administered intracerebroventricularly, resulted in poor memory retention in both the Morris water maze and elevated plus maze task paradigms and caused marked oxidative damage. It also caused a significant decrease in acetylcholinesterase activity. Naringin (40 and 80 mg/kg, p.o.) treatment was given daily for a period of 25 days beginning 4 days prior to colchicine administration. Chronic treatment with Naringin caused significant improvement in the cognitive performance and attenuated oxidative damage, as evidenced by lowering of malondialdehyde level and nitrite concentration and restoration of superoxide dismutase, catalase, glutathione S-transferase, and reduced glutathione levels, and acetylcholinesterase activity compared to control. The present study highlights the therapeutic potential of Naringin against colchicine-induced cognitive impairment and associated oxidative damage.
Antiulcer effect of naringin on gastric lesions induced by ethanol in rats.[Pubmed:7972328]
Pharmacology. 1994 Sep;49(3):144-50.
This study was designed to determine the gastroprotective properties of Naringin on and the involvement of endogenous prostaglandins in mucosal injury produced by absolute ethanol. Oral pretreatment with the highest dose of Naringin (400 mg/kg), 60 min before absolute ethanol was the most effective antiulcer treatment. Subcutaneous administration of indomethacin (10 mg/kg) to the animals treated with Naringin (400 mg/kg) partially inhibited gastric protection, but the prostaglandin E2 determination did not show any increase in prostanoid levels. The contents of gastric mucus and total proteins were not significantly modified. Naringin-treated rats showed a marked increase in hexosamine levels, but this increase was less in animals pretreated with indomethacin. These results show that Naringin has a 'cytoprotective' effect against ethanol injury in the rat, but this property appears to be mediated by non-prostaglandin-dependent mechanisms.
Naringin attenuates EGF-induced MUC5AC secretion in A549 cells by suppressing the cooperative activities of MAPKs-AP-1 and IKKs-IkappaB-NF-kappaB signaling pathways.[Pubmed:22766066]
Eur J Pharmacol. 2012 Sep 5;690(1-3):207-13.
Naringenin, the aglycone of Naringin, has been reported to attenuate MUC5AC secretion by inhibiting activity of nuclear factor kappa B (NF-kappaB) via EGFR-PI3K-Akt/ERK MAPKinase signaling pathways. However, previous studies demonstrated that the MUC5AC promoter was located in two different regions: an activator protein-1 (AP-1) binding site and a NF-kappaB binding site. The current study comprehensively determined the involvement of MAPKs/AP-1 and IKKs/IkappaB/NF-kappaB in epidermal growth factor (EGF)-induced A549 cells, and sought to ascertain the signaling pathways of Naringin imparted in suppression of EGF-induced MUC5AC secretion. The results showed that Naringin of 100 muM not only significantly decreased EGF-induced overexpressions of both MUC5AC mucin and mRNA in A549 cells, but also suppressed the phosphorylation of EGF receptor, p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK1/2), and c-Jun N-terminal kinase (JNK), as well as nucleus NF-kappaB p65 and AP-1. Moreover, any of three MAPKs inhibitors (PD98059, SB203580, and SP600125) significantly inhibited EGF-induced MUC5AC secretion. And as compared to MG132, the inhibitor kappaB (IkappaB) phosphorylation inhibitor of SN50 was more effective in reducing EGF-induced MUC5AC secretion because of suppression of nucleus AP-1. Meanwhile, as compared to Naringin, both SP600125 and azithromycin were less effective in suppressing EGF-induced secretion of MUC5AC because of the unchanged nucleus NF-kappaB p65. These results indicated that Naringin attenuates EGF-induced MUC5AC secretion in A549 cells by suppressing the cooperative activities of MAPKs/AP-1 and IKKs/IkappaB/NF-kappaB signaling pathways.
Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice.[Pubmed:17301733]
Clin Pharmacol Ther. 2007 Apr;81(4):495-502.
We showed previously that grapefruit and orange juices inhibited human enteric organic anion-transporting polypeptide (OATP)1A2 in vitro and lowered oral fexofenadine bioavailability clinically. Inhibition of OATP1A2 transport by flavonoids in grapefruit (Naringin) and orange (hesperidin) was conducted in vitro. Two randomized, crossover, pharmacokinetic studies were performed clinically. In one study, 120 mg of fexofenadine was ingested with 300 ml grapefruit juice, an aqueous solution of Naringin at the same juice concentration (1,200 microM), or water. In the other study, fexofenadine was administered with grapefruit juice, with or 2 h before aqueous suspension of the particulate fraction of juice containing known clinical inhibitors of enteric CYP3A4, but relatively low Naringin concentration (34 microM), or with water. Naringin and hesperidin's half-maximal inhibitions were 3.6 and 2.7 microM, respectively. Fexofenadine area under the plasma drug concentration-time curves (AUCs) with grapefruit juice and Naringin solution were 55% (P<0.001) and 75% (P<0.05) of that with water, respectively. Fexofenadine AUCs with grapefruit juice and particulate fractions were 57% (P<0.001), 96% (not significant (NS)), and 97% (NS) of that with water, respectively. Individuals tested in both studies (n=9 of 12) had highly reproducible fexofenadine AUC with water (r(2)=0.85, P<0.001) and extent of reduction of it with grapefruit juice (r(2)=0.72, P<0.01). Naringin most probably directly inhibited enteric OATP1A2 to decrease oral fexofenadine bioavailability. Inactivation of enteric CYP3A4 was probably not involved. Naringin appears to have sufficient safety, specificity, and sensitivity to be a clinical OATP1A2 inhibitor probe. Inherent OATP1A2 activity may be influenced by genetic factors. This appears to be the first report of a single dietary constituent clinically modulating drug transport.
Antihypercholesterolemic property of naringin alters plasma and tissue lipids, cholesterol-regulating enzymes, fecal sterol and tissue morphology in rabbits.[Pubmed:15380892]
Clin Nutr. 2004 Oct;23(5):1025-34.
BACKGROUND & AIMS: Hyperlipidemia is a major risk factor for cardiovascular diseases. This study was designed to confirm the hypocholesterolemic role of Naringin. METHODS: Male rabbits were fed 0.5% high-cholesterol diet or high-cholesterol diet supplemented with either 0.05% Naringin or 0.03% lovastatin for 8 weeks. RESULTS: The Naringin and lovastatin supplements significantly lowered plasma total- and LDL-cholesterol and hepatic lipids levels, while significantly increasing HDL-C/total-C ratio compared to the control group. Hepatic 3-hydroxy-3-methylglutaryl CoA reductase and acyl-CoA: cholesterol acyltransferase activities were significantly higher and lower, respectively, in both supplemented groups than the control group. Total fecal sterol content was significantly increased in lovastatin and especially Naringin group. In histopathological analyses, only control group exhibited hepatic lipid droplets, cardiac adipocyte infiltration and slight damage of endothelial lining in aortic wall, but two supplements retarded these atherogenic signs. CONCLUSION: It would appear that both Naringin and lovastatin contributed to hypocholesterolemic action via down-regulated ACAT activity and higher excretion of fecal sterols in response to high-cholesterol feeding. Also, Naringin supplement seemed to preserve tissue morphology from damages induced by high cholesterol diet.
Effects of naringin on hydrogen peroxide-induced cytotoxicity and apoptosis in P388 cells.[Pubmed:12832847]
J Pharmacol Sci. 2003 Jun;92(2):166-70.
Flavonoids are widely recognized as naturally occurring antioxidants. Naringin (NG) is one of the flavonoid components in citrus fruits such as grapefruit. Hydrogen peroxide (H2O2) causes cytotoxicity through oxidative stress and apoptosis. In this paper, we examined the effects of NG on H2O2-induced cytotoxicity and apoptosis in mouse leukemia P388 cells. Cytotoxicity was determined by mitochondrial activity (MTT assay). Apoptosis and DNA damage were analyzed by measuring chromatin condensation and Comet assay (alkaline single cell gel electrophoresis), respectively. H2O2-induced cytotoxicity was significantly attenuated by NG or the reduced form of glutathione (GSH), a typical intracellular antioxidant. NG suppressed chromatin condensation and DNA damage induced by H2O2. These results indicate that NG from natural products is a useful drug having antioxidant and anti-apoptopic properties.
Nitric oxide mechanism in the protective effect of naringin against post-stroke depression (PSD) in mice.[Pubmed:20433854]
Life Sci. 2010 Jun 19;86(25-26):928-35.
AIM: The present study has been designed to explore the nitric oxide mechanism in the protective effect of Naringin against I/R induced neurobehavioral alterations, oxidative damage and mitochondrial dysfunction in mice. MAIN METHODS: Laca mice (25-30 g) were subjected twice to BCCAO occlusion (5 min) at the interval of 10 min, followed by 96 h reperfusion. Naringin (50 and 100 mg/kg) was administered for 10 days, starting 7 days before the animals were subjected to I/R injury. On day 10, various neurobehavioral parameters followed by biochemical parameters and mitochondrial enzyme complex activities were assessed. KEY FINDINGS: Ischemia reperfusion injury caused significant (increased immobility period, neurological score and decreased locomotor activity) oxidative damage (increased lipid peroxidation and nitrite concentration and depleted reduced glutathione, glutathione-S-transferase, superoxide dismutase and catalase) and altered mitochondrial enzyme complex activities (complex I to IV) as compared to sham treatment. Naringin (50 and 100 mg/kg) treatment significantly attenuated neurobehavioral alterations, oxidative damage and restored mitochondrial enzyme complex activities as compared to control (ischemia reperfusion) group. Further, protective effect of Naringin (50 mg/kg) was attenuated by l-arginine (100 mg/kg) or sildenafil (5 mg/kg) pretreatment. Further, L-NAME (10 mg/kg) or 7-NI (10 mg/kg) pretreatment with Naringin (50 mg/kg) significantly potentiated their protective effect as compared to their treatment alone. SIGNIFICANCE: The present study suggests the involvement of nitric oxide mechanism in the protective effect of Naringin against post-stroke depression induced neurobehavioral, biochemical and cellular alterations in mice.
Naringin inhibits growth potential of human triple-negative breast cancer cells by targeting beta-catenin signaling pathway.[Pubmed:23694763]
Toxicol Lett. 2013 Jul 18;220(3):219-28.
Triple-negative (ER-/PR-/HER2-) breast cancer (TNBC) is a severe clinical problem because of its relatively poorer prognosis, aggressive behavior and lack of targeted therapies. Naringin, a major flavonoid extracted from citrus fruits, has been reported to exert promising anticancer activities. However, the detailed antitumor mechanism of Naringin still remains enigmatic. In this study, TNBC cell lines-based in vitro and in vivo models were used to explore the anticancer effect and mechanism of Naringin. Our data demonstrated that Naringin inhibited cell proliferation, and promoted cell apoptosis and G1 cycle arrest, accompanied by increased p21 and decreased survivin. Meanwhile, beta-catenin signaling pathway was found to be suppressed by Naringin. In contrast, over-expressing beta-catenin by adenoviral vector system in TNBC cells reversed the antitumor activity of Naringin, and regulated p21 and survivin. Correspondingly, the antitumor potential of Naringin was also observed in Naringin-treated MDA-MB-231 xenograft mice, while immunohistochemical analysis of tumors from Naringin-treated mice showed higher expression of p21 and lower expression of survivin and active beta-catenin. Taken together, these results indicate that Naringin could inhibit growth potential of TNBC cells by modulating beta-catenin pathway, which suggests Naringin might be used as a potential supplement for the prevention and treatment of breast cancer.


