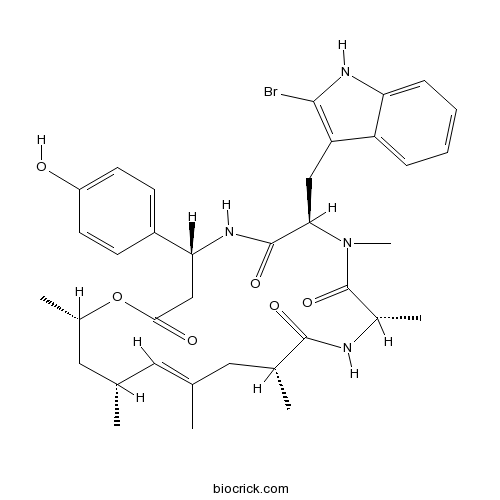
JasplakinolideStabilizes F-actin; promotes actin polymerization CAS# 102396-24-7 |
- CYT387 sulfate salt
Catalog No.:BCC1506
CAS No.:1056636-06-6
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- JAK2 Inhibitor V, Z3
Catalog No.:BCC1667
CAS No.:195371-52-9
- Bardoxolone methyl
Catalog No.:BCC1400
CAS No.:218600-53-4
- Ruxolitinib (INCB018424)
Catalog No.:BCC1276
CAS No.:941678-49-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 102396-24-7 | SDF | Download SDF |
| PubChem ID | 6436289 | Appearance | Powder |
| Formula | C36H45BrN4O6 | M.Wt | 709.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 2 mg/ml in DMSO | ||
| Chemical Name | (4R,7R,10S,13R,15E,17R,19S)-7-[(2-bromo-1H-indol-3-yl)methyl]-4-(4-hydroxyphenyl)-8,10,13,15,17,19-hexamethyl-1-oxa-5,8,11-triazacyclononadec-15-ene-2,6,9,12-tetrone | ||
| SMILES | CC1CC(OC(=O)CC(NC(=O)C(N(C(=O)C(NC(=O)C(CC(=C1)C)C)C)C)CC2=C(NC3=CC=CC=C32)Br)C4=CC=C(C=C4)O)C | ||
| Standard InChIKey | GQWYWHOHRVVHAP-HZIRKPFGSA-N | ||
| Standard InChI | InChI=1S/C36H45BrN4O6/c1-20-15-21(2)17-23(4)47-32(43)19-30(25-11-13-26(42)14-12-25)40-35(45)31(18-28-27-9-7-8-10-29(27)39-33(28)37)41(6)36(46)24(5)38-34(44)22(3)16-20/h7-15,21-24,30-31,39,42H,16-19H2,1-6H3,(H,38,44)(H,40,45)/b20-15+/t21-,22+,23-,24-,30+,31+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Rapidly stabilizes pre-formed actin filaments and inhibits their disassembly in vitro. Also induces polymerization of actin monomers into F-actin in vivo. Shown to bind to F-actin competitively with phalloidin (Kd ~ 15 nM). Exhibits antifungal and antiproliferative effects (IC50 = 35 nM for antiproliferative activity in PC3 cells). Cell permeable. |

Jasplakinolide Dilution Calculator

Jasplakinolide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4091 mL | 7.0455 mL | 14.0911 mL | 28.1821 mL | 35.2276 mL |
| 5 mM | 0.2818 mL | 1.4091 mL | 2.8182 mL | 5.6364 mL | 7.0455 mL |
| 10 mM | 0.1409 mL | 0.7046 mL | 1.4091 mL | 2.8182 mL | 3.5228 mL |
| 50 mM | 0.0282 mL | 0.1409 mL | 0.2818 mL | 0.5636 mL | 0.7046 mL |
| 100 mM | 0.0141 mL | 0.0705 mL | 0.1409 mL | 0.2818 mL | 0.3523 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AF-DX 116
Catalog No.:BCC6939
CAS No.:102394-31-0
- Glyburide
Catalog No.:BCC4784
CAS No.:10238-21-8
- Negsehisandrin G
Catalog No.:BCN2674
CAS No.:1023744-69-5
- Naringin
Catalog No.:BCN6312
CAS No.:10236-47-2
- Cadensin D
Catalog No.:BCN7260
CAS No.:102349-35-9
- (R)-Eriodictyol-8-C-beta-D-glucopyranoside
Catalog No.:BCN8028
CAS No.:1023271-51-3
- Epoxyparvinolide
Catalog No.:BCN5838
CAS No.:102227-61-2
- SGX-523
Catalog No.:BCC1055
CAS No.:1022150-57-7
- Btk inhibitor 1 R enantiomer
Catalog No.:BCC5125
CAS No.:1022150-12-4
- ARN2966
Catalog No.:BCC8074
CAS No.:102212-26-0
- Tyrosine kinase inhibitor
Catalog No.:BCC2020
CAS No.:1021950-26-4
- Boc-4-oxo-Pro-OMe
Catalog No.:BCC3436
CAS No.:102195-80-2
- Fmoc-Phg-OH
Catalog No.:BCC3312
CAS No.:102410-65-1
- RN 1747
Catalog No.:BCC7769
CAS No.:1024448-59-6
- Kushenol N
Catalog No.:BCN2984
CAS No.:102490-65-3
- GK921
Catalog No.:BCC8057
CAS No.:1025015-40-0
- Periglaucine A
Catalog No.:BCN5839
CAS No.:1025023-04-4
- Periglaucine B
Catalog No.:BCN7053
CAS No.:1025023-05-5
- SGI-1776 free base
Catalog No.:BCC2232
CAS No.:1025065-69-3
- (-)-Huperzine A
Catalog No.:BCN1057
CAS No.:102518-79-6
- 2,3,23-Trihydroxy-12-oleanen-28-oic acid
Catalog No.:BCN1638
CAS No.:102519-34-6
- R788 disodium
Catalog No.:BCC3695
CAS No.:1025687-58-4
- Methyl lucidente G
Catalog No.:BCN8269
CAS No.:102607-20-5
- Ganoderic acid L
Catalog No.:BCN8204
CAS No.:102607-24-9
Jasplakinolide reduces actin and tropomyosin dynamics during myofibrillogenesis.[Pubmed:25145272]
Cytoskeleton (Hoboken). 2014 Sep;71(9):513-29.
The premyofibril model proposes a three-stage process for the de novo assembly of myofibrils in cardiac and skeletal muscles: premyofibrils to nascent myofibrils to mature myofibrils. FRAP experiments and Jasplakinolide, a drug that stabilizes F-actin, permitted us to determine how decreasing the dynamics of actin filaments affected the dynamics of tropomyosin, troponin-T, troponin-C, and two Z-Band proteins (alpha-actinin, FATZ) in premyofibrils versus mature myofibrils. Jasplakinolide reduced markedly the dynamics of actin in premyofibrils and in mature myofibrils in skeletal muscles. Two isoforms of tropomyosin-1 (TPM1alpha, TPM1kappa) are more dynamic in premyofibrils than in mature myofibrils in control skeletal muscles. Jasplakinolide reduced the exchange rates of tropomyosins in premyofibrils but not in mature myofibrils. The reduced tropomyosin recoveries did not match the YFP-actin recoveries in premyofibrils in Jasplakinolide. There were no significant differences in the effects of Jasplakinolide on the dynamics of troponins in the thin filaments or of two Z-band proteins in premyofibrils or skeletal mature myofibrils. Cardiac control mature myofibrils lack nebulin, and small decreases in actin ( approximately 5%) and two tropomyosin isoforms ( approximately 10-15%) dynamics are detected in premyofibril to mature myofibril transformations compared with skeletal muscle. In contrast to skeletal muscle, Jasplakinolide lowered the dynamics of actin and tropomyosin isoforms in the cardiac mature myofibrils. These results suggest that the dynamics of tropomyosins in control muscle cells are related to actin exchange. These results also suggest a stabilizing role for nebulin, an actin and tropomyosin-binding protein, present in mature myofibrils but not in premyofibrils of skeletal muscles.
Actin-Dynamics in Plant Cells: The Function of Actin-Perturbing Substances: Jasplakinolide, Chondramides, Phalloidin, Cytochalasins, and Latrunculins.[Pubmed:26498789]
Methods Mol Biol. 2016;1365:243-61.
This chapter gives an overview of the most common F-actin-perturbing substances that are used to study actin dynamics in living plant cells in studies on morphogenesis, motility, organelle movement, or when apoptosis has to be induced. These substances can be divided into two major subclasses: F-actin-stabilizing and -polymerizing substances like Jasplakinolide and chondramides and F-actin-severing compounds like chytochalasins and latrunculins. Jasplakinolide was originally isolated form a marine sponge, and can now be synthesized and has become commercially available, which is responsible for its wide distribution as membrane-permeable F-actin-stabilizing and -polymerizing agent, which may even have anticancer activities. Cytochalasins, derived from fungi, show an F-actin-severing function and many derivatives are commercially available (A, B, C, D, E, H, J), also making it a widely used compound for F-actin disruption. The same can be stated for latrunculins (A, B), derived from red sea sponges; however the mode of action is different by binding to G-actin and inhibiting incorporation into the filament. In the case of swinholide a stable complex with actin dimers is formed resulting also in severing of F-actin. For influencing F-actin dynamics in plant cells only membrane permeable drugs are useful in a broad range. We however introduce also the phallotoxins and synthetic derivatives, as they are widely used to visualize F-actin in fixed cells. A particular uptake mechanism has been shown for hepatocytes, but has also been described in siphonal giant algae. In the present chapter the focus is set on F-actin dynamics in plant cells where alterations in cytoplasmic streaming can be particularly well studied; however methods by fluorescence applications including phalloidin and antibody staining as well as immunofluorescence-localization of the inhibitor drugs are given.
Actin stabilization by jasplakinolide affects the function of bone marrow-derived late endothelial progenitor cells.[Pubmed:23226422]
PLoS One. 2012;7(11):e50899.
BACKGROUND: Bone marrow-derived endothelial progenitor cells (EPCs), especially late EPCs, play a critical role in endothelial maintenance and repair, and postnatal vasculogenesis. Although the actin cytoskeleton has been considered as a modulator that controls the function and modulation of stem cells, its role in the function of EPCs, and in particular late EPCs, remains poorly understood. METHODOLOGY/PRINCIPAL FINDING: Bone marrow-derived late EPCs were treated with Jasplakinolide, a compound that stabilizes actin filaments. Cell apoptosis, proliferation, adhesion, migration, tube formation, nitric oxide (NO) production and endothelial NO synthase (eNOS) phosphorylation were subsequently assayed in vitro. Moreover, EPCs were locally infused into freshly balloon-injured carotid arteries, and the reendothelialization capacity was evaluated after 14 days. Jasplakinolide affected the actin distribution of late EPCs in a concentration and time dependent manner, and a moderate concentration of (100 nmol/l) Jasplakinolide directly stabilized the actin filament of late EPCs. Actin stabilization by Jasplakinolide enhanced the late EPC apoptosis induced by VEGF deprivation, and significantly impaired late EPC proliferation, adhesion, migration and tube formation. Furthermore, Jasplakinolide attenuated the reendothelialization capacity of transplanted EPCs in the injured arterial segment in vivo. However, eNOS phosphorylation and NO production were increased in late EPCs treated with Jasplakinolide. NO donor sodium nitroprusside (SNP) rescued the functional activities of Jasplakinolide-stressed late EPCs while the endothelial NO synthase inhibitor L-NAME led to a further dysfunction induced by Jasplakinolide in late EPCs. CONCLUSIONS/SIGNIFICANCE: A moderate concentration of Jasplakinolide results in an accumulation of actin filaments, enhancing the apoptosis induced by cytokine deprivation, and impairing the proliferation and function of late EPCs both in vitro and in vivo. NO donor reverses these impairments, suggesting the role of NO-related mechanisms in Jasplakinolide-induced EPC downregulation. Actin cytoskeleton may thus play a pivotal role in regulating late EPC function.
Tolerated doses in zebrafish of cytochalasins and jasplakinolide for comparison with tolerated doses in mice in the evaluation of pre-clinical activity of microfilament-directed agents in tumor model systems in vivo.[Pubmed:25398795]
In Vivo. 2014 Nov-Dec;28(6):1021-31.
BACKGROUND/AIM: Chemotherapeutic approaches involving microtubule-directed agents such as the vinca alkaloids and taxanes are used extensively and effectively in clinical cancer therapy. There is abundant evidence of critical cytoskeletal differences involving microfilaments between normal and neoplastic cells, and a variety of natural products and semi-synthetic derivatives are available to exploit these differences in vitro. In spite of the availability of such potential anti-neoplastic agents, there has yet to be an effective microfilament-directed agent approved for clinical use. Cytochalasins are mycogenic toxins derived from a variety of fungal sources that have shown promising in vitro efficacy in disrupting microfilaments and producing remarkable cell enlargement and multi-nucleation in cancer cells without producing enlargement and multi-nucleation in normal blood cells. Jasplakinolide is a sponge toxin that stabilizes and rigidifies microfilaments. Insufficient in vivo data has been acquired to determine whether any of the microfilament-directed agents have valuable preferential anticancer activity in pre-clinical tumor model systems. This is partly because the limited availability of these agents precludes their initial use in large-scale mammalian pre-clinical studies. Therefore, the present study sought to determine the tolerated in vivo doses of cytochalasins and Jasplakinolide in zebrafish (Danio rerio), a well-studied fish cancer model that is 1.5% the size of mice. We also determined the tolerated levels of a variety of clinically active anti-neoplastic agents in zebrafish for comparison with tolerated murine doses as a means to allow comparison of toxicities in zebrafish expressed as muM concentrations with toxicities in mice expressed in mg/kg. MATERIALS AND METHODS: Tolerated doses in zebrafish with various cytochalasins or Jasplakinolide were determined by adding the solubilized test agent to water in which the fish were maintained for 24 h, then restored to their normal tanks and monitored for a total of 96 h. RESULTS: Cytochalasin D at 0.2 muM gave an approximate LD50 in zebrafish, while cytochalasin B was fully-tolerated at 5 muM, and gave an LD50 of 10 muM. 21,22-dihydrocytochalasin B was fully-tolerated at 10 muM. Cytochalasin C was tolerated fully at 1 muM, ten-fold higher than the level for cytochalasin D that was tolerated. Jasplakinolide at 0.5 muM did not exhibit any apparent acute toxicity or affect fish behavior for four days, but delayed toxicity was evident at days 4 and 6 when the fish died. Further, the addition of 5 muM glutathione (GSH) at the time of treatment substantially decreased the toxicity of 10 muM cytochalasin B, a level of cytochalasin B that not otherwise tolerated in vivo. Such observations were likely due to GSH-mediated alkylation of C-20 in cytochalasin B, thereby reducing the rate of oxidation to the highly toxic congener, cytochalasin A, and reacting with any cytochalazin A formed. The protective effects of GSH are further supported by its ability to react with alpha, beta-unsaturated ketone moieties, as is found in cytochalasin A. GSH at 0.8 uM was able to reduce the toxicity of 0.8 muM cytochalasin D, but it took 20 muM GSH to fully protect against the toxicity of 0.8 muM cytochalasin D. CONCLUSION: Pre-clinical evaluation of rare natural products such as microfilamented-directed agents for efficacy in vivo in tumor-bearing zebrafish is a feasible prospect. Dose-limiting toxicities in zebrafish expressed as muM concentrations in water can be used to estimate in vivo toxicities in mice expressed as mg/kg.
Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations.[Pubmed:10671562]
J Biol Chem. 2000 Feb 18;275(7):5163-70.
Jasplakinolide paradoxically stabilizes actin filaments in vitro, but in vivo it can disrupt actin filaments and induce polymerization of monomeric actin into amorphous masses. A detailed analysis of the effects of Jasplakinolide on the kinetics of actin polymerization suggests a resolution to this paradox. Jasplakinolide markedly enhances the rate of actin filament nucleation. This increase corresponds to a change in the size of actin oligomer capable of nucleating filament growth from four to approximately three subunits, which is mechanistically consistent with the localization of the Jasplakinolide-binding site at an interface of three actin subunits. Because Jasplakinolide both decreases the amount of sequestered actin (by lowering the critical concentration of actin) and augments nucleation, the enhancement of polymerization by Jasplakinolide is amplified in the presence of actin-monomer sequestering proteins such as thymosin beta(4). Overall, the kinetic parameters in vitro define the mechanism by which Jasplakinolide induces polymerization of monomeric actin in vivo. Expected consequences of Jasplakinolide function are consistent with the experimental observations and include de novo nucleation resulting in disordered polymeric actin and in insufficient monomeric actin to allow for remodeling of stress fibers.
Role of actin-filament disassembly in lamellipodium protrusion in motile cells revealed using the drug jasplakinolide.[Pubmed:10531004]
Curr Biol. 1999 Oct 7;9(19):1095-105.
BACKGROUND: In motile cells, protrusion of the lamellipodium (a type of cell margin) requires assembly of actin monomers into actin filaments at the tip of the lamellipodium. The importance of actin-filament disassembly in this process is less well understood, and is assessed here using the actin drug Jasplakinolide, which has two known activities - inhibition of filament disassembly and induction of an increase in actin polymer. RESULTS: In cells the two activities of Jasplakinolide were found to be separable; 1 microM Jasplakinolide could permeate cells, bind cellular filamentous actin (F-actin) and inhibit filament disassembly within 3.5 minutes, but significant increase in actin polymer was not detected until 60 minutes of treatment. In live, permeabilised cells, Jasplakinolide did not inhibit filament assembly from supplied, purified actin monomers. In migrating chick fibroblasts, lamellipodium protrusion was blocked within 1-5 minutes of treatment with 1 microM Jasplakinolide, without any perturbation of actin organisation. In non-migrating chick fibroblasts, there was a delay in the onset of Jasplakinolide-induced inhibition of lamellipodium protrusion, during which lamellipodium length increased linearly with no increase in protrusion rate. Motility of the bacterium Listeria in infected PtK2 cells was reduced 2.3-fold within 3 minutes of treatment with 1 microM Jasplakinolide. CONCLUSIONS: Actin-filament disassembly is tightly coupled to lamellipodium protrusion in migrating chick fibroblasts and motility of Listeria in PtK2 cells. One simple interpretation of these data is a situation whereby ongoing actin-filament assembly uses free actin monomer derived from filament disassembly, in preference to stored monomer.
Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin.[Pubmed:8195116]
J Biol Chem. 1994 May 27;269(21):14869-71.
Jasplakinolide, a naturally occurring cyclic peptide from the marine sponge, Jaspis johnstoni, has both fungicidal and antiproliferative activity. We now report that this peptide is a potent inducer of actin polymerization in vitro. The peptide has a much greater effect on Mg(2+)-actin than on Ca(2+)-actin. Competitive binding studies using rhodamine-phalloidin suggest that Jasplakinolide binds to F-actin competitively with phalloidin with a dissociation constant of approximately 15 nM. This compares favorably to the previously reported IC50 of 35 nM for the antiproliferative effect of Jasplakinolide on PC3 prostate carcinoma cells. The binding curve suggests that nearest neighbor positive cooperativity influences the binding of Jasplakinolide (and perhaps also phalloidin) to F-actin. These results imply that Jasplakinolide may exert its cytotoxic effect in vivo by inducing actin polymerization and/or stabilizing pre-existing actin filaments.


