GK921CAS# 1025015-40-0 |
- Mycophenolate Mofetil
Catalog No.:BCC2290
CAS No.:128794-94-5
- AGI-5198
Catalog No.:BCC2293
CAS No.:1355326-35-0
- Trilostane
Catalog No.:BCC2302
CAS No.:13647-35-3
- AGI-6780
Catalog No.:BCC1331
CAS No.:1432660-47-3
- CPI-613
Catalog No.:BCC2287
CAS No.:95809-78-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1025015-40-0 | SDF | Download SDF |
| PubChem ID | 56682080 | Appearance | Powder |
| Formula | C21H20N4O | M.Wt | 344.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 30 mg/mL (87.11 mM) *"≥" means soluble, but saturation unknown. | ||
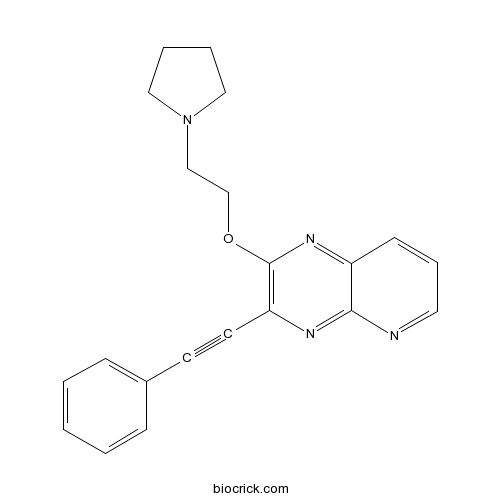
| Chemical Name | 3-(2-phenylethynyl)-2-(2-pyrrolidin-1-ylethoxy)pyrido[2,3-b]pyrazine | ||
| SMILES | C1CCN(C1)CCOC2=C(N=C3C(=N2)C=CC=N3)C#CC4=CC=CC=C4 | ||
| Standard InChIKey | MNYJJHBAEYKXEG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H20N4O/c1-2-7-17(8-3-1)10-11-19-21(26-16-15-25-13-4-5-14-25)24-18-9-6-12-22-20(18)23-19/h1-3,6-9,12H,4-5,13-16H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GK921 is a transglutaminase 2 (TGase) inhibitor with an IC50 of 7.71 μM for human recombinant TGase 2.In Vitro:GK921 inhibits the TGase 2-induced polymerization of I-κBα and p53 in a dose-dependent manner. The cytotoxicity of GK921 ranged from GI50 of 10-10 to 10-4 M. The average GI50 is 9.05×10-7 M. GK921 rescues p53 levels and consequently induces apoptosis; a concentration-dependent increase in cleaved poly(ADP-ribose) polymerase (c-PARP) and p53 levels is observed[1].In Vivo:A single treatment with GK921 almost completely reduces tumor growth by stabilizing p53 in the ACHN and CAKI-1 preclinical xenograft tumor models[1]. References: | |||||

GK921 Dilution Calculator

GK921 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9035 mL | 14.5176 mL | 29.0352 mL | 58.0703 mL | 72.5879 mL |
| 5 mM | 0.5807 mL | 2.9035 mL | 5.807 mL | 11.6141 mL | 14.5176 mL |
| 10 mM | 0.2904 mL | 1.4518 mL | 2.9035 mL | 5.807 mL | 7.2588 mL |
| 50 mM | 0.0581 mL | 0.2904 mL | 0.5807 mL | 1.1614 mL | 1.4518 mL |
| 100 mM | 0.029 mL | 0.1452 mL | 0.2904 mL | 0.5807 mL | 0.7259 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GK921 is a transglutaminase 2 (TGase 2) inhibitor with average GI50 of 0.9 uM in cancer cell lines.
- Kushenol N
Catalog No.:BCN2984
CAS No.:102490-65-3
- RN 1747
Catalog No.:BCC7769
CAS No.:1024448-59-6
- Fmoc-Phg-OH
Catalog No.:BCC3312
CAS No.:102410-65-1
- Jasplakinolide
Catalog No.:BCC7485
CAS No.:102396-24-7
- AF-DX 116
Catalog No.:BCC6939
CAS No.:102394-31-0
- Glyburide
Catalog No.:BCC4784
CAS No.:10238-21-8
- Negsehisandrin G
Catalog No.:BCN2674
CAS No.:1023744-69-5
- Naringin
Catalog No.:BCN6312
CAS No.:10236-47-2
- Cadensin D
Catalog No.:BCN7260
CAS No.:102349-35-9
- (R)-Eriodictyol-8-C-beta-D-glucopyranoside
Catalog No.:BCN8028
CAS No.:1023271-51-3
- Epoxyparvinolide
Catalog No.:BCN5838
CAS No.:102227-61-2
- SGX-523
Catalog No.:BCC1055
CAS No.:1022150-57-7
- Periglaucine A
Catalog No.:BCN5839
CAS No.:1025023-04-4
- Periglaucine B
Catalog No.:BCN7053
CAS No.:1025023-05-5
- SGI-1776 free base
Catalog No.:BCC2232
CAS No.:1025065-69-3
- (-)-Huperzine A
Catalog No.:BCN1057
CAS No.:102518-79-6
- 2,3,23-Trihydroxy-12-oleanen-28-oic acid
Catalog No.:BCN1638
CAS No.:102519-34-6
- R788 disodium
Catalog No.:BCC3695
CAS No.:1025687-58-4
- Methyl lucidente G
Catalog No.:BCN8269
CAS No.:102607-20-5
- Ganoderic acid L
Catalog No.:BCN8204
CAS No.:102607-24-9
- Saprorthoquinone
Catalog No.:BCN3147
CAS No.:102607-41-0
- Pantoprazole
Catalog No.:BCC5432
CAS No.:102625-70-7
- SC-9
Catalog No.:BCC6646
CAS No.:102649-78-5
- SC-10
Catalog No.:BCC6643
CAS No.:102649-79-6
Transglutaminase 2 inhibitor abrogates renal cell carcinoma in xenograft models.[Pubmed:24610445]
J Cancer Res Clin Oncol. 2014 May;140(5):757-67.
PURPOSE: To test whether transglutaminase 2 (TGase 2) inhibitor GK921 alone reverses renal cell carcinoma (RCC) tumor growth. RCC is resistant to both radiation and chemotherapy, and the prognosis remains poor. Despite the recent therapeutic success of vascular endothelial growth factor inhibition in RCC, approximately one-third of RCC patients develop metastatic disease. The expression of TGase 2 is markedly increased in most RCC cell lines, as well as in clinical samples. METHODS: Previously, we introduced the quinoxaline derivative GK13 as a lead compound for TGase 2 inhibitor. The inhibitory effect of GK13 on TGase 2 was improved in GK921 (3-(phenylethynyl)-2-(2-(pyridin-2-yl)ethoxy)pyrido[3,2-b]pyrazine). GK921 efficacy was tested using sulforhodamine in vitro as well as a xenograft tumor models using ACHN and CAKI-1 RCC cells. RESULTS: GK921 showed cytotoxicity to RCC (average GI50 in eight RCC cell lines: 0.905 muM). A single treatment with GK921 almost completely reduced tumor growth by stabilizing p53 in the ACHN and CAKI-1 preclinical xenograft tumor models. CONCLUSION: TGase 2 inhibitor GK921 abrogates RCC growth in xenograft tumor models, suggesting the possibility of a new therapeutic approach to RCC.
Transglutaminase 2 Inhibition Reverses Mesenchymal Transdifferentiation of Glioma Stem Cells by Regulating C/EBPbeta Signaling.[Pubmed:28754668]
Cancer Res. 2017 Sep 15;77(18):4973-4984.
Necrosis is a hallmark of glioblastoma (GBM) and is responsible for poor prognosis and resistance to conventional therapies. However, the molecular mechanisms underlying necrotic microenvironment-induced malignancy of GBM have not been elucidated. Here, we report that transglutaminase 2 (TGM2) is upregulated in the perinecrotic region of GBM and triggered mesenchymal (MES) transdifferentiation of glioma stem cells (GSC) by regulating master transcription factors (TF), such as C/EBPbeta, TAZ, and STAT3. TGM2 expression was induced by macrophages/microglia-derived cytokines via NF-kappaB activation and further degraded DNA damage-inducible transcript 3 (GADD153) to induce C/EBPbeta expression, resulting in expression of the MES transcriptome. Downregulation of TGM2 decreased sphere-forming ability, tumor size, and radioresistance and survival in a xenograft mouse model through a loss of the MES signature. A TGM2-specific inhibitor GK921 blocked MES transdifferentiation and showed significant therapeutic efficacy in mouse models of GSC. Moreover, TGM2 expression was significantly increased in recurrent MES patients and inversely correlated with patient prognosis. Collectively, our results indicate that TGM2 is a key molecular switch of necrosis-induced MES transdifferentiation and an important therapeutic target for MES GBM. Cancer Res; 77(18); 4973-84. (c)2017 AACR.


