PantoprazoleH+/K+-ATPase inhibitor CAS# 102625-70-7 |
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 102625-70-7 | SDF | Download SDF |
| PubChem ID | 4679 | Appearance | Powder |
| Formula | C16H15F2N3O4S | M.Wt | 383.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >18mg/mL in DMSO | ||
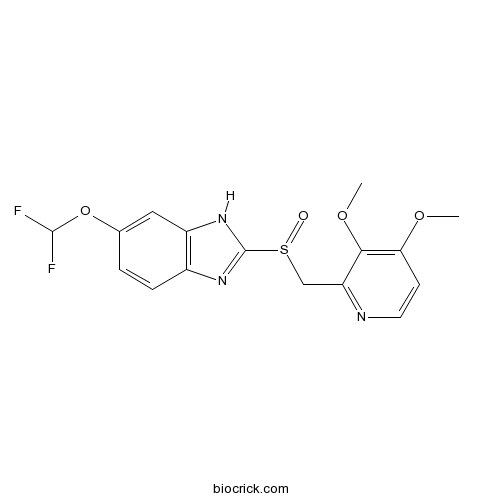
| Chemical Name | 6-(difluoromethoxy)-2-[(3,4-dimethoxypyridin-2-yl)methylsulfinyl]-1H-benzimidazole | ||
| SMILES | COC1=C(C(=NC=C1)CS(=O)C2=NC3=C(N2)C=C(C=C3)OC(F)F)OC | ||
| Standard InChIKey | IQPSEEYGBUAQFF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H15F2N3O4S/c1-23-13-5-6-19-12(14(13)24-2)8-26(22)16-20-10-4-3-9(25-15(17)18)7-11(10)21-16/h3-7,15H,8H2,1-2H3,(H,20,21) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Pantoprazole Dilution Calculator

Pantoprazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6084 mL | 13.0422 mL | 26.0845 mL | 52.1689 mL | 65.2112 mL |
| 5 mM | 0.5217 mL | 2.6084 mL | 5.2169 mL | 10.4338 mL | 13.0422 mL |
| 10 mM | 0.2608 mL | 1.3042 mL | 2.6084 mL | 5.2169 mL | 6.5211 mL |
| 50 mM | 0.0522 mL | 0.2608 mL | 0.5217 mL | 1.0434 mL | 1.3042 mL |
| 100 mM | 0.0261 mL | 0.1304 mL | 0.2608 mL | 0.5217 mL | 0.6521 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pantoprazole is an inhibitor of H+/K+-ATPase [1].
Pantoprazole inhibits the activity of H+/K+-ATPase proton pumb in the parietal cells of gastric mucosa. This inhibition affects the acid secretion and thus, pantoprazole are used as drugs for the treatment of various acid-related disorders. Pantoprazole is activated slowly. The activated sulfonamide of pantoprazole binds to Cys813 and Cys822 of the pumb and inhibits acid secretion selectively. Pantoprazole inhibited lysosomal acidification with IC50 value of 194 μM. The pKa values of pantoprazole are 3.83 (pKa1) and 0.11 (pKa2). Besides that, pantoprazole also shows antibacterial effects on Helicobacter pylori [1, 2].
References:
[1] Jungnickel P W. Pantoprazole: a new proton pump inhibitor. Clinical therapeutics, 2000, 22(11): 1268-1293.
[2] Sachs G, Shin J M, Howden C W. Review article: the clinical pharmacology of proton pump inhibitors. Alimentary pharmacology & therapeutics, 2006, 23(s2): 2-8.
- Saprorthoquinone
Catalog No.:BCN3147
CAS No.:102607-41-0
- Ganoderic acid L
Catalog No.:BCN8204
CAS No.:102607-24-9
- Methyl lucidente G
Catalog No.:BCN8269
CAS No.:102607-20-5
- R788 disodium
Catalog No.:BCC3695
CAS No.:1025687-58-4
- 2,3,23-Trihydroxy-12-oleanen-28-oic acid
Catalog No.:BCN1638
CAS No.:102519-34-6
- (-)-Huperzine A
Catalog No.:BCN1057
CAS No.:102518-79-6
- SGI-1776 free base
Catalog No.:BCC2232
CAS No.:1025065-69-3
- Periglaucine B
Catalog No.:BCN7053
CAS No.:1025023-05-5
- Periglaucine A
Catalog No.:BCN5839
CAS No.:1025023-04-4
- GK921
Catalog No.:BCC8057
CAS No.:1025015-40-0
- Kushenol N
Catalog No.:BCN2984
CAS No.:102490-65-3
- RN 1747
Catalog No.:BCC7769
CAS No.:1024448-59-6
- SC-9
Catalog No.:BCC6646
CAS No.:102649-78-5
- SC-10
Catalog No.:BCC6643
CAS No.:102649-79-6
- RO-3
Catalog No.:BCC7548
CAS No.:1026582-88-6
- LB-100
Catalog No.:BCC5532
CAS No.:1026680-07-8
- Labd-13-ene-8,15-diol
Catalog No.:BCN5840
CAS No.:10267-31-9
- VX-222 (VCH-222, Lomibuvir)
Catalog No.:BCC2108
CAS No.:1026785-59-0
- H-Gln(Trt)-OH
Catalog No.:BCC2919
CAS No.:102747-84-2
- PSB 0788
Catalog No.:BCC7599
CAS No.:1027513-54-7
- Levetiracetam
Catalog No.:BCC1056
CAS No.:102767-28-2
- GYKI 52466 dihydrochloride
Catalog No.:BCC7072
CAS No.:102771-26-6
- [D-p-Cl-Phe6,Leu17]-VIP
Catalog No.:BCC5968
CAS No.:102805-45-8
- RuBi-GABA
Catalog No.:BCC6012
CAS No.:1028141-88-9
The effect of short-term oral treatment with omeprazole or pantoprazole on the function of polymorphonuclear neutrophils.[Pubmed:28177671]
Can J Physiol Pharmacol. 2017 Jun;95(6):675-680.
Recent studies report an increased risk of enteric infections in patients treated with proton pump inhibitors (PPIs). Polymorphonuclear neutrophils (PMNs) play a key role in host response to bacterial infection. We evaluated the effect of omeprazole and Pantoprazole treatment on the PMN function. Fifteen patients were treated with omeprazole 20 mg daily and 15 patients with Pantoprazole 40 mg daily for 7 days. Treatment with omeprazole or Pantoprazole had no effect on spontaneous nitroblue tetrazolium (NBT) test results. Significant increase in the percentage of phagocytes in the omeprazole group in stimulated NBT test (by 69%) was found. Treatment with omeprazole or Pantoprazole had no effect on nitric oxide (NO) concentration in the PMN culture supernatant and serum, cyclic guanosine monophosphate concentration in the PMN culture supernatant and serum, as well as inducible nitric oxide synthase (iNOS) protein expression and p38 mitogen-activated protein kinase activity in PMNs. In conclusion, treatment with PPI has no effect on NO production and p38 mitogen-activated protein kinase activity in PMNs. Interestingly, short-term treatment with omeprazole but not with Pantoprazole enhances PMN reactive oxygen species production.
A chiral LC-MS/MS method for the enantioselective determination of R-(+)- and S-(-)-pantoprazole in human plasma and its application to a pharmacokinetic study of S-(-)-pantoprazole sodium injection.[Pubmed:28370240]
Biomed Chromatogr. 2017 Oct;31(10).
Pantoprazole, a proton pump inhibitor, is clinically used for the treatment of peptic diseases. An enantioselective LC-MS/MS method was developed and validated for the simultaneous determination of Pantoprazole enantiomers in human plasma. Pantoprazole enantiomers and the internal standard were extracted from plasma using acetonitrile. Chiral separation was carried on a Chiralpak IE column using the mobile phase consisted of 10 mm ammonium acetate solution containing 0.1% acetic acid-acetonitrile (28 : 72, v/v). MS analysis was performed on an API 4000 mass spectrometer. Multiple reactions monitoring transitions of m/z 384.1-->200.1 and 390.1-->206.0 were used to quantify Pantoprazole enantiomers and internal standard, respectively. For each enantiomer, no apparent matrix effect was found, the calibration curve was linear over 5.00-10,000 ng/mL, the intra- and inter-day precisions were below 10.0%, and the accuracy was within the range of -5.6% to 0.6%. This method was applied to the stereoselective pharmacokinetic studies in human after intravenous administration of S-(-)-Pantoprazole sodium injections. No chiral inversion was observed during sample storage, preparation procedure and analysis. While R-(+)-Pantoprazole was detected in human plasma with a slightly high concentration, which implied that S-(-)-Pantoprazole may convert to R-(+)-Pantoprazole in some subjects.
Up-regulation of autophagy is a mechanism of resistance to chemotherapy and can be inhibited by pantoprazole to increase drug sensitivity.[Pubmed:28378028]
Cancer Chemother Pharmacol. 2017 May;79(5):959-969.
BACKGROUND: Autophagy is a survival mechanism that allows recycling of cellular breakdown products, particularly in stressed cells. Here we evaluate the hypotheses that up-regulation of autophagy is a common mechanism of resistance to chemotherapy, and that drug resistance can be reversed by inhibiting autophagy with a proton pump inhibitor. METHODS: We exposed human PC3, LNCaP and MCF7 cells to seven clinically-used chemotherapy drugs +/- Pantoprazole, examined the up-regulation of autophagy and the effect on cellular proliferation by Western Blots, MTS assay and colony-forming assay. The distribution of drug effects and of autophagy was quantified in LNCaP tumor sections in relation to blood vessels and hypoxia by immunohistochemistry using gammaH2AX, cleaved caspase-3 and p62. RESULTS: All anticancer drugs led to up-regulation of autophagy in cultured tumor cells. Pantoprazole inhibited the induction of autophagy in a time- and dose-dependent manner, and sensitized cancer cells to the seven anti-cancer drugs. Treatment of LNCaP xenografts with paclitaxel induced both DNA damage and autophagy; autophagy was inhibited and markers of toxicity were increased by Pantoprazole. CONCLUSIONS: Induction of autophagy is a general mechanism associated with resistance to anticancer drugs and that its inhibition is a promising therapeutic strategy to enhance the effects of chemotherapy and improve clinical outcomes.


