GYKI 52466 dihydrochlorideCAS# 102771-26-6 |
- Pioglitazone HCl
Catalog No.:BCC2278
CAS No.:112529-15-4
- Rosiglitazone
Catalog No.:BCC2264
CAS No.:122320-73-4
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- T0070907
Catalog No.:BCC2261
CAS No.:313516-66-4
- Troglitazone
Catalog No.:BCC2016
CAS No.:97322-87-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 102771-26-6 | SDF | Download SDF |
| PubChem ID | 3538 | Appearance | Powder |
| Formula | C17H15N3O2 | M.Wt | 293.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO and to 10 mM in water | ||
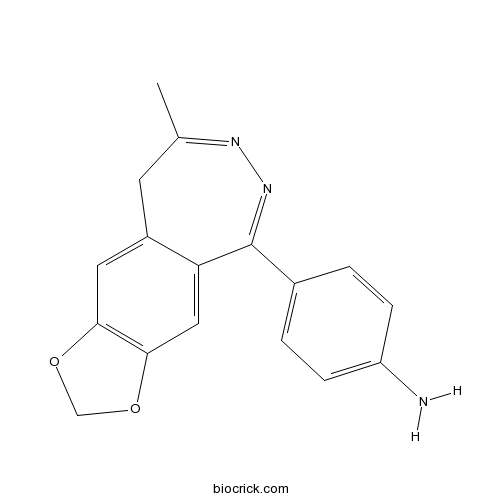
| Chemical Name | 4-(8-methyl-9H-[1,3]dioxolo[4,5-h][2,3]benzodiazepin-5-yl)aniline | ||
| SMILES | CC1=NN=C(C2=CC3=C(C=C2C1)OCO3)C4=CC=C(C=C4)N | ||
| Standard InChIKey | LFBZZHVSGAHQPP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H15N3O2/c1-10-6-12-7-15-16(22-9-21-15)8-14(12)17(20-19-10)11-2-4-13(18)5-3-11/h2-5,7-8H,6,9,18H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective non-competitive AMPA receptor antagonist (IC50 values are 10-20, ~ 450 and >> 50 μM for AMPA- , kainate- and NMDA-induced responses respectively). Skeletal muscle relaxant and orally-active anticonvulsant. Has anti-proliferative effects in transformed cells. Also available as part of the AMPA Receptor. Also available as part of the Kainate Receptor. |

GYKI 52466 dihydrochloride Dilution Calculator

GYKI 52466 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4095 mL | 17.0474 mL | 34.0948 mL | 68.1896 mL | 85.237 mL |
| 5 mM | 0.6819 mL | 3.4095 mL | 6.819 mL | 13.6379 mL | 17.0474 mL |
| 10 mM | 0.3409 mL | 1.7047 mL | 3.4095 mL | 6.819 mL | 8.5237 mL |
| 50 mM | 0.0682 mL | 0.3409 mL | 0.6819 mL | 1.3638 mL | 1.7047 mL |
| 100 mM | 0.0341 mL | 0.1705 mL | 0.3409 mL | 0.6819 mL | 0.8524 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Levetiracetam
Catalog No.:BCC1056
CAS No.:102767-28-2
- PSB 0788
Catalog No.:BCC7599
CAS No.:1027513-54-7
- H-Gln(Trt)-OH
Catalog No.:BCC2919
CAS No.:102747-84-2
- VX-222 (VCH-222, Lomibuvir)
Catalog No.:BCC2108
CAS No.:1026785-59-0
- Labd-13-ene-8,15-diol
Catalog No.:BCN5840
CAS No.:10267-31-9
- LB-100
Catalog No.:BCC5532
CAS No.:1026680-07-8
- RO-3
Catalog No.:BCC7548
CAS No.:1026582-88-6
- SC-10
Catalog No.:BCC6643
CAS No.:102649-79-6
- SC-9
Catalog No.:BCC6646
CAS No.:102649-78-5
- Pantoprazole
Catalog No.:BCC5432
CAS No.:102625-70-7
- Saprorthoquinone
Catalog No.:BCN3147
CAS No.:102607-41-0
- Ganoderic acid L
Catalog No.:BCN8204
CAS No.:102607-24-9
- [D-p-Cl-Phe6,Leu17]-VIP
Catalog No.:BCC5968
CAS No.:102805-45-8
- RuBi-GABA
Catalog No.:BCC6012
CAS No.:1028141-88-9
- Dihydrocinchonamine
Catalog No.:BCN5841
CAS No.:10283-68-8
- VUF 10460
Catalog No.:BCC6285
CAS No.:1028327-66-3
- BEZ235 Tosylate
Catalog No.:BCC1416
CAS No.:1028385-32-1
- D-Pinitol
Catalog No.:BCN5842
CAS No.:10284-63-6
- Mulberroside A
Catalog No.:BCN6343
CAS No.:102841-42-9
- Mulberroside C
Catalog No.:BCN6344
CAS No.:102841-43-0
- Moracin P
Catalog No.:BCN3289
CAS No.:102841-46-3
- Ganolactone B
Catalog No.:BCN2872
CAS No.:1028449-53-7
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- Intermedine
Catalog No.:BCN1997
CAS No.:10285-06-0
Glutamate antagonists limit tumor growth.[Pubmed:11331750]
Proc Natl Acad Sci U S A. 2001 May 22;98(11):6372-7.
Neuronal progenitors and tumor cells possess propensity to proliferate and to migrate. Glutamate regulates proliferation and migration of neurons during development, but it is not known whether it influences proliferation and migration of tumor cells. We demonstrate that glutamate antagonists inhibit proliferation of human tumor cells. Colon adenocarcinoma, astrocytoma, and breast and lung carcinoma cells were most sensitive to the antiproliferative effect of the N-methyl-d-aspartate antagonist dizocilpine, whereas breast and lung carcinoma, colon adenocarcinoma, and neuroblastoma cells responded most favorably to the alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate antagonist GYKI52466. The antiproliferative effect of glutamate antagonists was Ca(2+) dependent and resulted from decreased cell division and increased cell death. Morphological alterations induced by glutamate antagonists in tumor cells consisted of reduced membrane ruffling and pseudopodial protrusions. Furthermore, glutamate antagonists decreased motility and invasive growth of tumor cells. These findings suggest anticancer potential of glutamate antagonists.
Comparison of anticonvulsive and acute neuroprotective activity of three 2,3-benzodiazepine compounds, GYKI 52466, GYKI 53405, and GYKI 53655.[Pubmed:11489346]
Brain Res Bull. 2001 Jun;55(3):387-91.
GYKI 52466 [1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine], a non-competitive AMPA [alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate] and kainate receptor antagonist and its two analogues, GYKI 53405 [1-(4-aminophenyl)-3-acetyl-4-methyl-3,4-dihydro-7,8-methylenedioxy-5H-2,3-benzod iazepine] and GYKI 53655 [1-(4-aminophenyl)-3-methylcarbamyl-4-methyl-3,4-dihydro-7,8-methylenedioxy-5H-2, 3-benzodiazepine] were investigated in two seizure models and in MgCl2 induced global cerebral ischaemia, as an acute neuroprotective model. The ED(50) values of GYKI 52466 for suppression of the tonic and clonic phases of sound-induced seizures were 3.6 and 4.3 mg/kg, respectively. The corresponding data for GYKI 53405 were 1.1 and 3.1 mg/kg, while ED(50) values of GYKI 53655 were 1.3 and 2.0 mg/kg, respectively. The inhibition of seizure evoked by maximal electroshock was also found to be remarkable: the ED(50) values of GYKI 52466 and its two analogues were 6.9, 2.6, and 2.2 mg/kg, respectively. All compounds prolonged the survival times in MgCl2 induced global cerebral ischaemia test in a dose-dependent fashion, with PD(50) (dose of 50% prolongation) values of 24.1, 8.3, and 8.2 mg/kg intraperitoneal, respectively. In audiogenic seizure model the duration of anticonvulsant action of 10 mg/kg GYKI 52466 and 5 mg/kg GYKI 53405, GYKI 53655 were examined, too. The effect of GYKI 52466 decreased to 50% after 2 h, while the analogues showed more than 80% seizure suppression 3 h after treatment. After 6 h the effect of GYKI 53655 decreased to zero, while the effect of GYKI 52466, remained on the 50% level.
Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons.[Pubmed:7826635]
Neuron. 1995 Jan;14(1):185-9.
Although both protein and mRNAs for kainate receptor subunits are abundant in several brain regions, the responsiveness of AMPA receptors to kainate has made it difficult to demonstrate the presence of functional kainate-type receptors in native cells. Recently, however, we have shown that many hippocampal neurons in culture express glutamate receptors of the kainate type. The large nondesensitizing response that kainate induces at AMPA receptors precludes detection and analysis of smaller, rapidly desensitizing currents induced by kainate at kainate receptors. Consequently, the functional significance of these strongly desensitizing glutamate receptors remains enigmatic. We report here that the family of new noncompetitive antagonists of AMPA receptors (GYKI 52466 and 53655) minimally affects kainate-induced responses at kainate receptors while completely blocking AMPA receptor-mediated currents, making it possible to separate the responses mediated by each receptor. These compounds will allow determination of the role played by kainate receptors in synaptic transmission and plasticity in the mammalian brain, as well as evaluation of their involvement in neurotoxicity.
GYKI 52466, a 2,3-benzodiazepine, is a highly selective, noncompetitive antagonist of AMPA/kainate receptor responses.[Pubmed:7678966]
Neuron. 1993 Jan;10(1):51-9.
In whole-cell voltage-clamp recordings from cultured rat hippocampal neurons, the 2,3-benzodiazepine GYKI 52466 was a potent antagonist of kainate- and AMPA-activated currents (IC50 values, 7.5 and 11 microM, respectively), but was inactive against N-methyl-D-aspartate (NMDA) or gamma-aminobutyric acid responses. The block produced by GYKI 52466 occurred in a noncompetitive fashion, was voltage independent, and failed to show use dependence, indicating an allosteric blocking mechanism. In kinetic experiments with kainate as the agonist, the GYKI 52466 binding and unbinding rates were 1.6 x 10(5) M-1 s-1 and 3.2 s-1, respectively. GYKI 52466 also suppressed non-NMDA receptor-mediated spontaneous synaptic currents via a postsynaptic action. Non-competitive AMPA/kainate antagonists such as GYKI 52466 could offer advantages over competitive antagonists in the treatment of glutamate-associated neurological disorders, particularly under conditions in which high levels of the amino acid would render the competitive antagonists relatively ineffective. Moreover, the results demonstrate the existence of a novel recognition site for an atypical benzodiazepine on non-NMDA receptors.
Electrophysiological studies with a 2,3-benzodiazepine muscle relaxant: GYKI 52466.[Pubmed:2574112]
Eur J Pharmacol. 1989 Aug 22;167(2):193-9.
The effects of GYKI 52466, a new 2,3-benzodiazepine with muscle relaxant and anticonvulsant properties, were investigated and compared to those of midazolam in electrophysiological experiments. The effects of the drugs on the reflex potentials evoked by afferent nerve stimulation and recorded from the spinal roots in unanesthetized spinal cats were studied. GYKI 52466 exerted a strong inhibitory effect on the monosynaptic as well as the polysynaptic ventral root reflexes, while the dorsal root responses decreased slightly. In contrast, midazolam markedly enhanced the dorsal root responses, did not modify the monosynaptic reflex and partially inhibited the polysynaptic reflex. The spontaneous firing of cerebellar Purkinje cells was depressed by midazolam, but not by GYKI 52466. These results suggest strongly that, contrary to the classical 1,4-benzodiazepines, potentiation of the GABA-A receptor-mediated inhibition does not play a significant role in the pharmacological actions of GYKI 52466.


