RO-3Selective P2X3 and P2X2/3 antagonist CAS# 1026582-88-6 |
- AVL-292
Catalog No.:BCC1385
CAS No.:1202757-89-8
- QL47
Catalog No.:BCC3920
CAS No.:1469988-75-7
- PCI 29732
Catalog No.:BCC4100
CAS No.:330786-25-9
- CGI-1746
Catalog No.:BCC1473
CAS No.:910232-84-7
- PCI-32765 (Ibrutinib)
Catalog No.:BCC1266
CAS No.:936563-96-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1026582-88-6 | SDF | Download SDF |
| PubChem ID | 11289644 | Appearance | Powder |
| Formula | C16H22N4O2 | M.Wt | 302.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
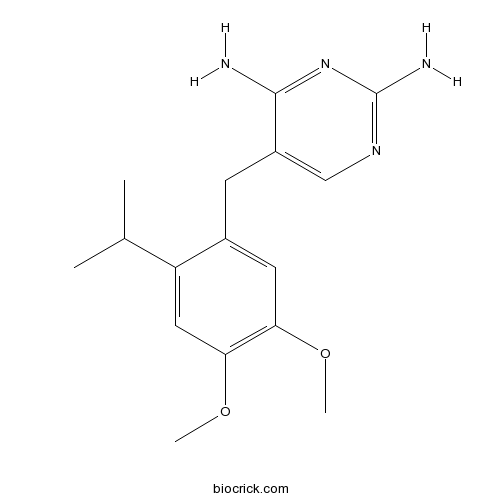
| Chemical Name | 5-[(4,5-dimethoxy-2-propan-2-ylphenyl)methyl]pyrimidine-2,4-diamine | ||
| SMILES | CC(C)C1=CC(=C(C=C1CC2=CN=C(N=C2N)N)OC)OC | ||
| Standard InChIKey | PYNPWUIBJMVRIG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H22N4O2/c1-9(2)12-7-14(22-4)13(21-3)6-10(12)5-11-8-19-16(18)20-15(11)17/h6-9H,5H2,1-4H3,(H4,17,18,19,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective homomeric P2X3 and heteromeric P2X2/3 receptor antagonist (pIC50 values are 7.0 and 5.9 respectively) that exhibits no activity at P2X1, P2X2, P2X4, P2X5 and P2X7 receptors (IC50 > 10 μM). Attenuates nociceptive sensitivity in animal models of pain. Orally active and brain penetrant. |

RO-3 Dilution Calculator

RO-3 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3072 mL | 16.536 mL | 33.0721 mL | 66.1441 mL | 82.6802 mL |
| 5 mM | 0.6614 mL | 3.3072 mL | 6.6144 mL | 13.2288 mL | 16.536 mL |
| 10 mM | 0.3307 mL | 1.6536 mL | 3.3072 mL | 6.6144 mL | 8.268 mL |
| 50 mM | 0.0661 mL | 0.3307 mL | 0.6614 mL | 1.3229 mL | 1.6536 mL |
| 100 mM | 0.0331 mL | 0.1654 mL | 0.3307 mL | 0.6614 mL | 0.8268 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SC-10
Catalog No.:BCC6643
CAS No.:102649-79-6
- SC-9
Catalog No.:BCC6646
CAS No.:102649-78-5
- Pantoprazole
Catalog No.:BCC5432
CAS No.:102625-70-7
- Saprorthoquinone
Catalog No.:BCN3147
CAS No.:102607-41-0
- Ganoderic acid L
Catalog No.:BCN8204
CAS No.:102607-24-9
- Methyl lucidente G
Catalog No.:BCN8269
CAS No.:102607-20-5
- R788 disodium
Catalog No.:BCC3695
CAS No.:1025687-58-4
- 2,3,23-Trihydroxy-12-oleanen-28-oic acid
Catalog No.:BCN1638
CAS No.:102519-34-6
- (-)-Huperzine A
Catalog No.:BCN1057
CAS No.:102518-79-6
- SGI-1776 free base
Catalog No.:BCC2232
CAS No.:1025065-69-3
- Periglaucine B
Catalog No.:BCN7053
CAS No.:1025023-05-5
- Periglaucine A
Catalog No.:BCN5839
CAS No.:1025023-04-4
- LB-100
Catalog No.:BCC5532
CAS No.:1026680-07-8
- Labd-13-ene-8,15-diol
Catalog No.:BCN5840
CAS No.:10267-31-9
- VX-222 (VCH-222, Lomibuvir)
Catalog No.:BCC2108
CAS No.:1026785-59-0
- H-Gln(Trt)-OH
Catalog No.:BCC2919
CAS No.:102747-84-2
- PSB 0788
Catalog No.:BCC7599
CAS No.:1027513-54-7
- Levetiracetam
Catalog No.:BCC1056
CAS No.:102767-28-2
- GYKI 52466 dihydrochloride
Catalog No.:BCC7072
CAS No.:102771-26-6
- [D-p-Cl-Phe6,Leu17]-VIP
Catalog No.:BCC5968
CAS No.:102805-45-8
- RuBi-GABA
Catalog No.:BCC6012
CAS No.:1028141-88-9
- Dihydrocinchonamine
Catalog No.:BCN5841
CAS No.:10283-68-8
- VUF 10460
Catalog No.:BCC6285
CAS No.:1028327-66-3
- BEZ235 Tosylate
Catalog No.:BCC1416
CAS No.:1028385-32-1
Antagonism with dibenamine, D-600, and Ro 3-7894 to estimate dissociation constants and receptor reserves for cardiac adrenoceptors in isolated rabbit papillary muscles.[Pubmed:6290018]
Can J Physiol Pharmacol. 1982 Aug;60(8):1131-7.
In papillary muscles isolated from reserpinized rabbits, positive inotropic responses to the alpha (alpha)-adrenergic agonist, (-)-phenylephrine in the presence of 10(7) M timolol and the beta (beta)-adrenergic agonist. (-)-isoproterenol were antagonized with the irreversible alpha-adrenergic antagonist, dibenamine, the irreversible beta-adrenergic antagonist. Ro 3-7894, and the calcium blocker, D-600. D-600 was employed as a functional antagonist of both alpha- and beta-adrenoceptor responses. Dissociation constants (Ka values) for drug-receptor interactions were calculated by the method of Furchgott and used to estimate fractional receptor occupancy and agonist efficacies. Comparison of responses showed that the receptor reserve for cardiac beta-adrenoceptors was greater than for alpha-adrenoceptors. D-600 was an effective inhibitor of both cardiac alpha- and beta-adrenoceptor responses; however, estimates of KA and receptor reserves were similar to estimates using an irreversible antagonist for alpha-but not beta-adrenoceptors.
[Simultaneous improvement of mood and release of growth hormone by L-5-hydroxytryptophan (Ro 3-5940) in normal subjects (author's transl)].[Pubmed:313797]
Arzneimittelforschung. 1978;28(8):1291-2.
Infusion of a new soluble ester of L-5-hydroxytryptophan produced euphoria in 34/35 experiments in healthy subjects. Parallel measurements of growth hormone and mood changes showed a similar rise and fall of these two parameters in 8/11 subjects. These results indicate that central stimulatory serotoninergic mechanisms (in addition to the well-known dopaminergic and alpha-adrenergic stimulation) play a role in the control of growth hormone release.
Ro 3-4787, a new beta-adrenoceptor blocking agent: Studies in normal volunteers.[Pubmed:22454901]
Br J Clin Pharmacol. 1974 Apr;1(2):143-9.
1 A new beta-adrenoceptor blocking agent, Ro 3-4787, was administered orally to resting subjects. Peak levels of 106.2 +/- 15.5 ng/ml were reached after 20 mg given in the fasting state. After intravenous administration, an inverse rectilinear relationship was shown between the log dose (2 mg-20 mg) and the heart rate during vigorous exercise on a bicycle ergometer. 2 A double-blind study in five normal subjects using 0.03 mg/kg and 0.15 mg/kg showed that propranolol and Ro 3-4787 were equally effective in reducing the exercise heart rate. There was an inverse correlation between the log plasma level before exercise and the heart rate during exercise for both drugs over the range studied.
Painful purinergic receptors.[Pubmed:18042830]
J Pharmacol Exp Ther. 2008 Feb;324(2):409-15.
Multiple P2 receptor-mediated mechanisms exist by which ATP can alter nociceptive sensitivity following tissue injury. Evidence from a variety of experimental strategies, including genetic disruption studies and the development of selective antagonists, has indicated that the activation of P2X receptor subtypes, including P2X(3), P2X(2/3), P2X(4) and P2X(7), and P2Y (e.g., P2Y(2)) receptors, can modulate pain. For example, administration of a selective P2X(3) antagonist, A-317491, has been shown to effectively block both hyperalgesia and allodynia in different animal models of pathological pain. Intrathecally delivered antisense oligonucleotides targeting P2X(4) receptors decrease tactile allodynia following nerve injury. Selective antagonists for the P2X(7) receptor also reduce sensitization in animal models of inflammatory and neuropathic pain, providing evidence that purinergic glial-neural interactions are important modulators of noxious sensory neurotransmission. Furthermore, activation of P2Y(2) receptors leads to sensitization of polymodal transient receptor potential-1 receptors. Thus, ATP acting at multiple purinergic receptors, either directly on neurons (e.g., P2X(3), P2X(2/3), and P2Y receptors) or indirectly through neural-glial cell interactions (P2X(4) and P2X(7) receptors), alters nociceptive sensitivity. The development of selective antagonists for some of these P2 receptors has greatly aided investigations into the nociceptive role of ATP. This perspective highlights some of the recent advances to identify selective P2 receptor ligands, which has enhanced the investigation of ATP-related modulation of pain sensitivity.
Purinoceptors as therapeutic targets for lower urinary tract dysfunction.[Pubmed:16465177]
Br J Pharmacol. 2006 Feb;147 Suppl 2:S132-43.
Lower urinary tract symptoms (LUTS) are present in many common urological syndromes. However, their current suboptimal management by muscarinic and alpha(1)-adrenoceptor antagonists leaves a significant opportunity for the discovery and development of superior medicines. As potential targets for such therapeutics, purinoceptors have emerged over the last two decades from investigations that have established a prominent role for ATP in the regulation of urinary bladder function under normal and pathophysiological conditions. In particular, evidence suggests that ATP signaling via P2X(1) receptors participates in the efferent control of detrusor smooth muscle excitability, and that this function may be heightened in disease and aging. ATP also appears to be involved in bladder sensation, via activation of P2X(3) and P2X(2/3) receptors on sensory afferent neurons, both within the bladder itself and possibly at central synapses. Such findings are based on results from classical pharmacological and localization studies in non-human and human tissues, knockout mice, and studies using recently identified pharmacological antagonists--some of which possess attributes that offer the potential for optimization into candidate drug molecules. Based on recent advances in this field, it is clearly possible that the development of selective antagonists for these receptors will occur that could lead to therapies offering better relief of sensory and motor symptoms for patients, while minimizing the systemic side effects that limit current medicines.


