NimodipineCa2+ channel blocker (L-type) CAS# 66085-59-4 |
- Vinblastine Sulfate
Catalog No.:BCN2292
CAS No.:143-67-9
- Pepstatin A
Catalog No.:BCC1218
CAS No.:26305-03-3
- Dexamethasone (DHAP)
Catalog No.:BCC1184
CAS No.:50-02-2
- Omeprazole
Catalog No.:BCC1254
CAS No.:73590-58-6
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 66085-59-4 | SDF | Download SDF |
| PubChem ID | 4497 | Appearance | Powder |
| Formula | C21H26N2O7 | M.Wt | 418.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BAY-e 9736 | ||
| Solubility | DMSO : 100 mg/mL (238.98 mM; Need ultrasonic) | ||
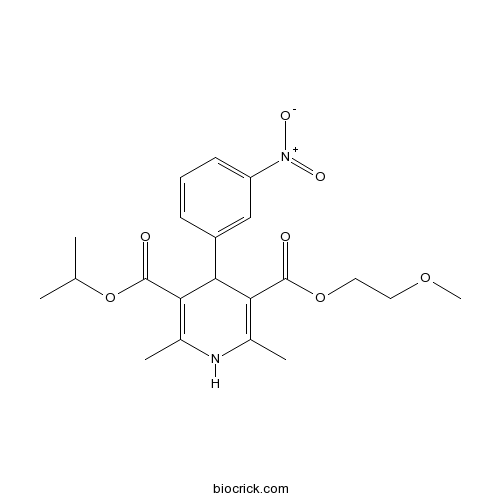
| Chemical Name | 3-O-(2-methoxyethyl) 5-O-propan-2-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate | ||
| SMILES | CC1=C(C(C(=C(N1)C)C(=O)OC(C)C)C2=CC(=CC=C2)[N+](=O)[O-])C(=O)OCCOC | ||
| Standard InChIKey | UIAGMCDKSXEBJQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H26N2O7/c1-12(2)30-21(25)18-14(4)22-13(3)17(20(24)29-10-9-28-5)19(18)15-7-6-8-16(11-15)23(26)27/h6-8,11-12,19,22H,9-10H2,1-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | L-type Ca2+ channel blocker. |

Nimodipine Dilution Calculator

Nimodipine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3898 mL | 11.9489 mL | 23.8977 mL | 47.7954 mL | 59.7443 mL |
| 5 mM | 0.478 mL | 2.3898 mL | 4.7795 mL | 9.5591 mL | 11.9489 mL |
| 10 mM | 0.239 mL | 1.1949 mL | 2.3898 mL | 4.7795 mL | 5.9744 mL |
| 50 mM | 0.0478 mL | 0.239 mL | 0.478 mL | 0.9559 mL | 1.1949 mL |
| 100 mM | 0.0239 mL | 0.1195 mL | 0.239 mL | 0.478 mL | 0.5974 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nimodipine is an L-type calcium channel protein inhibitor. Nimodipine is an inhibitor of Mdr.
- GW 542573X
Catalog No.:BCC7914
CAS No.:660846-41-3
- Tigloylgomisin H
Catalog No.:BCN6927
CAS No.:66069-55-4
- Licoisoflavanone
Catalog No.:BCN6856
CAS No.:66067-26-3
- Licoisoflavone B
Catalog No.:BCN6695
CAS No.:66056-30-2
- Benzoylgomisin H
Catalog No.:BCN7242
CAS No.:66056-23-3
- Angeloylgomisin H
Catalog No.:BCN2843
CAS No.:66056-22-2
- Gomisin H
Catalog No.:BCN3902
CAS No.:66056-20-0
- Licoisoflavone A
Catalog No.:BCN2929
CAS No.:66056-19-7
- Glycyrin
Catalog No.:BCN7681
CAS No.:66056-18-6
- H-D-Arg(NO2)-OH
Catalog No.:BCC2871
CAS No.:66036-77-9
- (5Z)-7-Oxozeaenol
Catalog No.:BCC7724
CAS No.:66018-38-0
- 6-Demethoxytangeretin
Catalog No.:BCN3844
CAS No.:6601-66-7
- GW843682X
Catalog No.:BCC1614
CAS No.:660868-91-7
- Docosanol (Abreua)
Catalog No.:BCC3769
CAS No.:661-19-8
- Glochidone
Catalog No.:BCN4207
CAS No.:6610-55-5
- Glochidiol
Catalog No.:BCN4208
CAS No.:6610-56-6
- Pergolide mesylate
Catalog No.:BCC4464
CAS No.:66104-23-2
- 14,17-Epidioxy-28-nor-15-taraxerene-2,3-diol
Catalog No.:BCN1386
CAS No.:66107-60-6
- Levalbuterol tartrate
Catalog No.:BCC4217
CAS No.:661464-94-4
- Chromolaenide
Catalog No.:BCN7341
CAS No.:66148-25-2
- Cimicifugoside
Catalog No.:BCN2623
CAS No.:66176-93-0
- Malic acid 4-Me ester
Catalog No.:BCN4209
CAS No.:66178-02-7
- Deacetylxylopic acid
Catalog No.:BCN4210
CAS No.:6619-95-0
- Cyromazine
Catalog No.:BCC5328
CAS No.:66215-27-8
Prophylactic nimodipine treatment and improvement in hearing outcome after vestibular schwannoma surgery: a combined analysis of a randomized, multicenter, Phase III trial and its pilot study.[Pubmed:28298021]
J Neurosurg. 2017 Dec;127(6):1376-1383.
OBJECTIVE In clinical routines, neuroprotective strategies in neurosurgical interventions are still missing. A pilot study (n = 30) and an analogously performed Phase III trial (n = 112) pointed to a beneficial effect of prophylactic Nimodipine and hydroxyethyl starch (HES) in vestibular schwannoma (VS) surgery. Considering the small sample size, the data from both studies were pooled. METHODS The patients in both investigator-initiated studies were assigned to 2 groups. The treatment group (n = 70) received parenteral Nimodipine (1-2 mg/hour) and HES (hematocrit 30%-35%) from the day before surgery until the 7th postoperative day. The control group (n = 72) was not treated prophylactically. Facial and cochlear nerve functions were documented preoperatively, during the inpatient care, and 1 year after surgery. RESULTS Pooled raw data were analyzed retrospectively. Intent-to-treat analysis revealed a significantly lower risk for hearing loss (Class D) 12 months after surgery in the treatment group compared with the control group (OR 0.46, 95% CI 0.22-0.97; p = 0.04). After exclusion of patients with preoperative Class D hearing, this effect was more pronounced (OR 0.38, 95% CI 0.17-0.83; p = 0.016). Logistic regression analysis adjusted for tumor size showed a 4 times lower risk for hearing loss in the treatment group compared with the control group (OR 0.25, 95% CI 0.09-0.63; p = 0.003). Facial nerve function was not significantly improved with treatment. Apart from dose-dependent hypotension (p < 0.001), the study medication was well tolerated. CONCLUSIONS Prophylactic Nimodipine is safe and may be recommended in VS surgery to preserve hearing. Prophylactic neuroprotective treatment in surgeries in which nerves are at risk seems to be a novel and promising concept. Clinical trial registration no.: DRKS 00000328 ( https://drks-neu.uniklinik-freiburg.de/drks_web/ ).
Systemic and Cerebral Concentration of Nimodipine During Established and Experimental Vasospasm Treatment.[Pubmed:28344178]
World Neurosurg. 2017 Jun;102:459-465.
BACKGROUND: Oral Nimodipine is an established prophylactic agent for cerebral vasospasm after subarachnoid hemorrhage (SAH). In highly selected cases, intra-arterial (IA) or intravenous (IV) application of Nimodipine may be considered; however, the optimum dosage and modality of application remain a matter of debate. The purpose of this investigation is analysis of Nimodipine concentration in serum, cerebrospinal fluid, and cerebral microdialysate in the context of currently effective dose and route of application (oral, IA, IV). METHODS: We prospectively collected 156 samples from 37 patients treated for aneurysmal SAH from May 2014 to July 2015. Treatment groups were stratified according to modality of application and low-dose or high-dose treatment. At time of sampling, current dose and modality of application effectively sustained cerebral perfusion as documented by common diagnostics. Samples were analyzed for Nimodipine concentration via high-performance liquid chromatography and tandem mass spectrometry. RESULTS: In most cases (94.3%), Nimodipine remained below the limit of quantification (0.5 ng/mL) within the brain (microdialysis, cerebrospinal fluid), even during targeted, local application (IA Nimodipine). The median serum concentration for all treatment groups was 17.3 ng/mL. Modality of application (oral, IA, IV) was not associated with significant differences in serum concentrations (P = 0.712), even after stratification for dosage (P = 0.371), implying a comparable systemic distribution, if not efficacy. CONCLUSIONS: Nimodipine does not accumulate sufficiently within the target organ for treatment monitoring. Comparable systemic concentrations can be observed irrespective of application modality and dosing. Future studies will clarify the role of efficacy-driven treatment algorithms, in which lowest dose and least invasive mode of application still effective should be identified.
Chemical modulators of autophagy as biological probes and potential therapeutics.[Pubmed:21164513]
Nat Chem Biol. 2011 Jan;7(1):9-17.
Autophagy is an evolutionarily conserved mechanism for protein degradation that is critical for the maintenance of homeostasis in man. Autophagy has unexpected pleiotropic functions that favor survival of the cell, including nutrient supply under starvation, cleaning of the cellular interior, defense against infection and antigen presentation. Moreover, defective autophagy is associated with a diverse range of disease states, including neurodegeneration, cancer and Crohn's disease. Here we discuss the roles of mammalian autophagy in health and disease and highlight recent advances in pharmacological manipulation of autophagic pathways as a therapeutic strategy for a variety of pathological conditions.
Beneficial effect of the Ca2+ antagonist, nimodipine, on existing diabetic neuropathy in the BB/Wor rat.[Pubmed:8019766]
Br J Pharmacol. 1994 Mar;111(3):887-93.
1. Neuropathy is a frequently diagnosed complication of diabetes mellitus. Effective pharmacotherapy is not available. 2. The spontaneously diabetic BB/Wor rats develop secondary complications like neuropathy as do human diabetic patients. 3. BB/Wor rats treated with insulin via a subcutaneous implant show a significant impairment of sensory and motor nerve conduction velocity 6 weeks after the onset of diabetes mellitus. 4. Intraperitoneal treatment of diabetic BB/Wor rats with the Ca2+ antagonist, Nimodipine (20 mg kg-1), from week 6 onwards every 48 h for a period of 6 weeks resulted in a significant increase of sensory and motor nerve conduction velocity. 5. Twelve weeks after the onset of diabetes mellitus BB/Wor rats show a 40% impairment of sciatic nerve blood flow as compared to the non-diabetic age-matched controls. Treatment with Nimodipine (20 mg kg-1) from week 6 onwards significantly increased the sciatic nerve blood flow as compared to placebo-treated diabetic BB/Wor rats. 6. The adrenergic responsiveness of the vasa nervorum of the sciatic nerve to tyramine and phenylephrine was investigated as a parameter for autonomic neuropathy. 7. The fact that Nimodipine treatment restored the reduced response to tyramine independently of the reduced postsynaptic phenylephrine responsiveness indicates that Nimodipine improves adrenergic responsiveness mainly at the presynaptic level.


