NitrocefinUsed for detection of β-lactamases CAS# 41906-86-9 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 41906-86-9 | SDF | Download SDF |
| PubChem ID | 6436140 | Appearance | Powder |
| Formula | C21H16N4O8S2 | M.Wt | 516.50 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
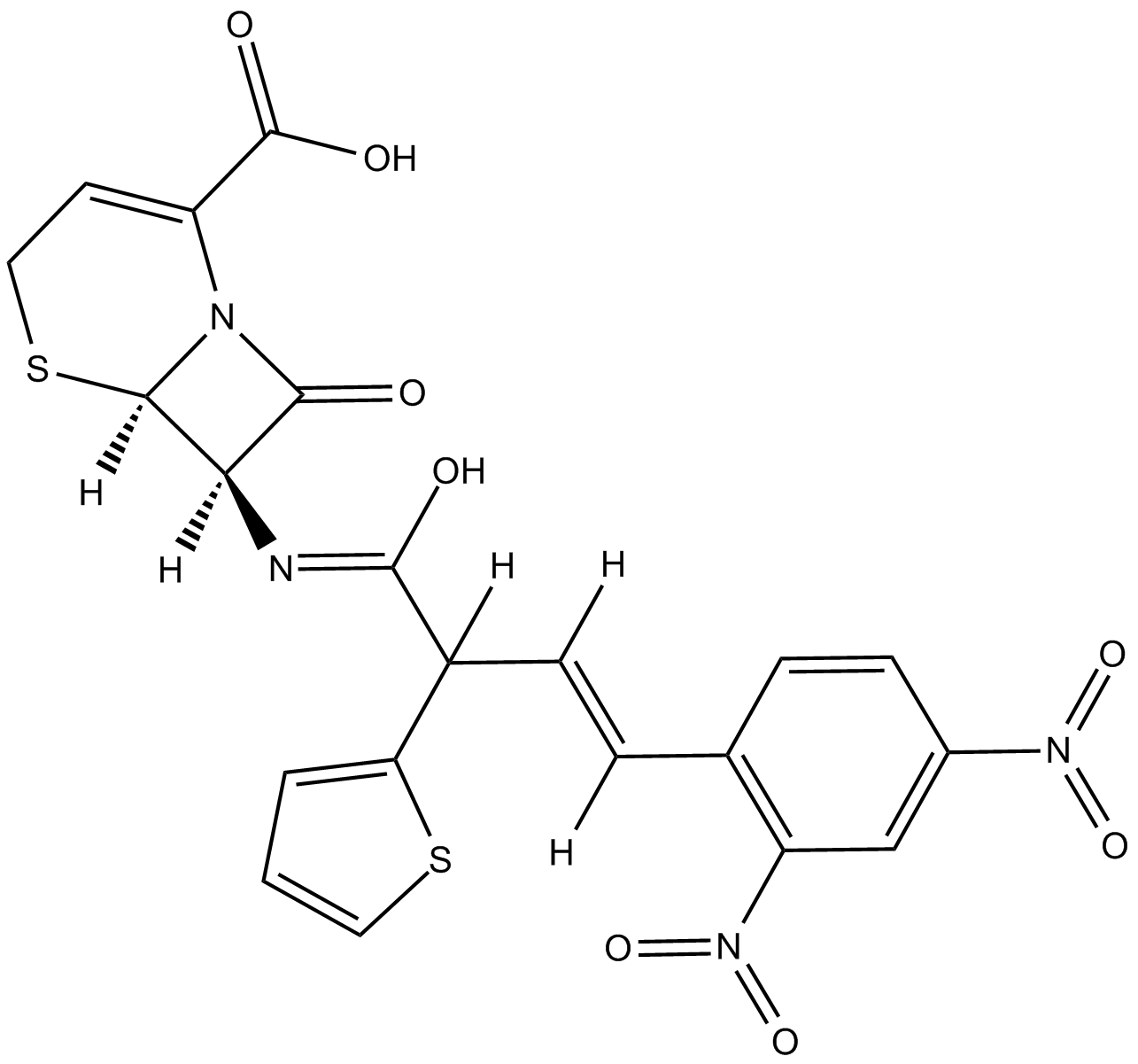
| Chemical Name | (6R,7R)-3-[(E)-2-(2,4-dinitrophenyl)ethenyl]-8-oxo-7-[(2-thiophen-2-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | ||
| SMILES | C1C(=C(N2C(S1)C(C2=O)NC(=O)CC3=CC=CS3)C(=O)O)C=CC4=C(C=C(C=C4)[N+](=O)[O-])[N+](=O)[O-] | ||
| Standard InChIKey | LHNIIDJCEODSHA-OQRUQETBSA-N | ||
| Standard InChI | InChI=1S/C21H16N4O8S2/c26-16(9-14-2-1-7-34-14)22-17-19(27)23-18(21(28)29)12(10-35-20(17)23)4-3-11-5-6-13(24(30)31)8-15(11)25(32)33/h1-8,17,20H,9-10H2,(H,22,26)(H,28,29)/b4-3+/t17-,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Nitrocefin Dilution Calculator

Nitrocefin Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nitrocefin is a chromogenic cephalosporin substrate which is used for detection of β-lactamases. β-lactamases are enzymes produced by various microbes that mediate resistance to β-lactam antibiotics such as penicillins and cephamycins. Comparing to other methods for β-lactamases detection, nitrocefin is more sensitive while using few materials and inexpensive equipment. The color change by nitrocefin can be followed quantitatively by measuring changes in absorption between 380 to 500 nm region[1].
Reference:
[1] O'Callaghan, Cynthia H et al. Novel Method for Detection of B-Lactamases by Using a Chromogenic Cephalosporin Substrate. Antimicrobial Agents and Chemotherapy. 1972 Apr; 1(4):283-288.
- PYR-41
Catalog No.:BCC4470
CAS No.:418805-02-4
- 2,3-DCPE hydrochloride
Catalog No.:BCC2384
CAS No.:418788-90-6
- Bezafibrate
Catalog No.:BCC4639
CAS No.:41859-67-0
- Alatamine
Catalog No.:BCN3100
CAS No.:41855-33-8
- Mangiferolic acid
Catalog No.:BCN4636
CAS No.:4184-34-3
- Hesperetin-7-methyl ether
Catalog No.:BCN8501
CAS No.:
- Junicedric acid
Catalog No.:BCN7660
CAS No.:41787-69-3
- Hypecorinine
Catalog No.:BCN3298
CAS No.:41787-57-9
- H-Leu-pNA.HCl
Catalog No.:BCC2972
CAS No.:4178-93-2
- Lenvatinib (E7080)
Catalog No.:BCC1172
CAS No.:417716-92-8
- 14-Deoxyandrographolide
Catalog No.:BCN3706
CAS No.:4176-97-0
- ar-Curcumene
Catalog No.:BCN7534
CAS No.:4176-06-1
- Isopropyl 4-Hydroxybenzoate
Catalog No.:BCN8409
CAS No.:4191-73-5
- Trilobatin
Catalog No.:BCN5479
CAS No.:4192-90-9
- N1-Methyl-4-nitrobenzene-1,2-diamine
Catalog No.:BCC9070
CAS No.:41939-61-1
- MLR 1023
Catalog No.:BCC6232
CAS No.:41964-07-2
- Pinocembrin chalcone
Catalog No.:BCN7223
CAS No.:4197-97-1
- Glabranin
Catalog No.:BCN5480
CAS No.:41983-91-9
- (S)-(-)-Propranolol hydrochloride
Catalog No.:BCC6809
CAS No.:4199-10-4
- 5-Chloro-1,10-phenanthroline
Catalog No.:BCC3713
CAS No.:4199-89-7
- Dihydropashanone
Catalog No.:BCN4635
CAS No.:41997-41-5
- Antitumor Compound 1
Catalog No.:BCC5397
CAS No.:420126-30-3
- Fenofibric acid
Catalog No.:BCC8982
CAS No.:42017-89-0
- Estriol 17-sulfate
Catalog No.:BCN2237
CAS No.:42028-21-7
Kinetic characterization of hydrolysis of nitrocefin, cefoxitin, and meropenem by beta-lactamase from Mycobacterium tuberculosis.[Pubmed:23672214]
Biochemistry. 2013 Jun 11;52(23):4097-104.
The constitutively expressed, chromosomally encoded beta-lactamase (BlaC) is the enzyme responsible for the intrinsic resistance to beta-lactam antibiotics in Mycobacterium tuberculosis. Previous studies from this laboratory have shown that the enzyme exhibits an extended-spectrum phenotype, with very high levels of penicillinase and cephalosporinase activity, as well as weak carbapenemase activity [Tremblay, L. W., et al. (2008) Biochemistry 47, 5312-5316]. In this report, we have determined the pH dependence of the kinetic parameters, revealing that the maximal velocity depends on the ionization state of two groups: a general base exhibiting a pK value of 4.5 and a general acid exhibiting a pK value of 7.8. Having defined a region where the kinetic parameters are pH-independent (pH 6.5), we determined solvent kinetic isotope effects (SKIEs) for three substrates whose kcat values differ by 5.5 orders of magnitude. Nitrocefin is a highly activated, chromogenic cephalosporin derivative that exhibits steady-state solvent kinetic isotope effects of 1.4 on both V and V/K. Cefoxitin is a slower cephalosporin derivative that exhibits a large SKIE on V of 3.9 but a small SKIE of 1.8 on V/K in steady-state experiments. Pre-steady-state, stopped-flow experiments with cefoxitin revealed a burst of beta-lactam ring opening with associated SKIE values of 1.6 on the acylation step and 3.4 on the deacylation step. Meropenem is an extremely slow substrate for BlaC and exhibits burst kinetics in the steady-state experiments. SKIE determinations with meropenem revealed large SKIEs on both the acylation and deacylation steps of 3.8 and 4.0, respectively. Proton inventories in all cases were linear, indicating the participation of a single solvent-derived proton in the chemical step responsible for the SKIE. The rate-limiting steps for beta-lactam hydrolysis of these substrates are analyzed, and the chemical steps responsible for the observed SKIE are discussed.
A novel family VIII carboxylesterase derived from a leachate metagenome library exhibits promiscuous beta-lactamase activity on nitrocefin.[Pubmed:19190902]
Appl Microbiol Biotechnol. 2009 Jun;83(3):491-500.
The realization that majority of microbes are not amenable to cultivation as isolates under laboratory conditions has led to the culture-independent metagenomic approach as a novel technique for novel biocatalyst discovery. A leachate fosmid shotgun metagenome library was constructed and subsequently screened for esterolytic activities on a tributyrin agar medium. Nucleotide sequencing and translational analysis of an esterase-positive fosmid clone led to the identification of a 1,281 bp esterase gene (estC) encoding a protein (EstC) of 427 aa with translated molecular weight of 46.3 kDa. The EstC primary structure contained a signal leader peptide (29 aa), which could be cleaved to form a mature protein of 398 aa with molecular weight 43.3 kDa. Homology searches revealed that EstC belonged to the family VIII esterases, which exploit a serine residue within the S-x-x-K motif as a catalytic nucleophile. Substrate specificity studies showed that EstC prefers short to medium acyl chain length of p-nitrophenyl esters, a characteristic typical of "true" carboxylesterases. Moreover, EstC represents the first member of the family VIII esterases with a leader peptide and a detectable promiscuous beta-lactam hydrolytic activity. Site-directed mutagenesis studies also revealed that in addition to Ser103 and Lys106 residues, the Tyr219 residue also plays a catalytic role in EstC. The organic solvent stability and the specificity towards esters of tertiary alcohols linalyl acetate (3,7-dimethyl-1,6-octadien-3-yl acetate) make EstC potentially useful in biocatalysis.
A nitrocefin-based amperometric assay for the rapid quantification of extended-spectrum beta-lactamase-producing Escherichia coli in wastewaters.[Pubmed:27951476]
Water Res. 2017 Feb 1;109:375-381.
A sensitive and inexpensive amperometric assay based on the electrochemical detection of the beta-lactamase activity using the Nitrocefin as substrate was developed for the rapid and quantitative detection of extended spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) in urban wastewaters. The specific detection of ESBL-EC was achieved by culturing the filtered sample in a medium containing the cefotaxime supplemented or not with the potassium clavulanate inhibitor. This step was followed by the incubation of each subculture filtrate with the Nitrocefin substrate which hydrolysis was monitored by amperometry using disposable carbon screen-printed sensors. Current intensities iCef and iClav correspond to the intensity of the anodic current measured ( approximately + 0.2 V vs. Ag/AgCl) for the sample incubated with the cefotaxime without and with potassium clavulanate, respectively. The intensity value i = iCef - iClav was chosen as the analytical response. ESBL-EC calibration plots were established with artificially contaminated wastewater samples. This assay allowed the detection of ESBL-EC amounts as low as 10 cfu in treated effluents and 100 cfu in raw wastewaters with short time analysis of 5.5 h and 4.5 h, respectively. The amperometric method was applied to the analysis of 38 wastewater samples and the results were in good agreement with CFU counts on a selective chromogenic medium for 24 h. Owing to its rapidity, convenience, low-cost and portability, this assay is a promising tool to obtain quantitative data on antimicrobial-resistant E. coli in wastewater effluents. Furthermore, this assay might be used to improve wastewater treatment plant processes in order to minimize the release of antibiotic resistant bacteria into the aquatic environment.
Assay for drug discovery: Synthesis and testing of nitrocefin analogues for use as beta-lactamase substrates.[Pubmed:26142222]
Anal Biochem. 2015 Oct 1;486:75-7.
We report on the synthesis of three Nitrocefin analogues and their evaluation as substrates for the detection of beta-lactamase activity. These compounds are hydrolyzed by all four Ambler classes of beta-lactamases. Kinetic parameters were determined with eight different beta-lactamases, including VIM-2, NDM-1, KPC-2, and SPM-1. The compounds do not inhibit the growth of clinically important antibiotic-resistant gram-negative bacteria in vitro. These chromogenic compounds have a distinct absorbance spectrum and turn purple when hydrolyzed by beta-lactamases. One of these compounds, UW154, is easier to synthesize from commercial starting materials than Nitrocefin and should be significantly less expensive to produce.


