BezafibrateCAS# 41859-67-0 |
- BEZ235 Tosylate
Catalog No.:BCC1416
CAS No.:1028385-32-1
- GSK2126458
Catalog No.:BCC3884
CAS No.:1086062-66-9
- VS-5584 (SB2343)
Catalog No.:BCC2047
CAS No.:1246560-33-7
- Everolimus (RAD001)
Catalog No.:BCC3594
CAS No.:159351-69-6
- PIK-294
Catalog No.:BCC4995
CAS No.:900185-02-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 41859-67-0 | SDF | Download SDF |
| PubChem ID | 39042 | Appearance | Powder |
| Formula | C19H20ClNO4 | M.Wt | 361.82 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BM15075 | ||
| Solubility | DMSO : ≥ 50 mg/mL (138.19 mM) *"≥" means soluble, but saturation unknown. | ||
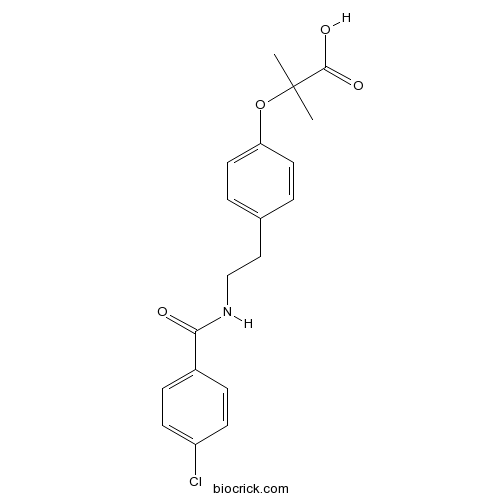
| Chemical Name | 2-[4-[2-[(4-chlorobenzoyl)amino]ethyl]phenoxy]-2-methylpropanoic acid | ||
| SMILES | CC(C)(C(=O)O)OC1=CC=C(C=C1)CCNC(=O)C2=CC=C(C=C2)Cl | ||
| Standard InChIKey | IIBYAHWJQTYFKB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H20ClNO4/c1-19(2,18(23)24)25-16-9-3-13(4-10-16)11-12-21-17(22)14-5-7-15(20)8-6-14/h3-10H,11-12H2,1-2H3,(H,21,22)(H,23,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bezafibrate is an agonist of PPAR, with EC50s of 50 μM, 60 μM, 20 μM for human PPARα, PPARγ and PPARδ, and 90 μM, 55 μM, 110 μM for murine PPARα, PPARγ and PPARδ, respectively; Bezafibrate is used as an hypolipidemic agent.In Vitro:Bezafibrate is an agonist of PPAR, with EC50s of 90 μM, 55 μM, 110 μM for murine PPARα, PPARγ and PPARδ, and 50 μM, 60 μM, 20 μM for human PPARα, PPARγ and PPARδ, respectively[1]. Bezafibrate (> 200 μM) shows significant cytotoxicity against human retinal microvascular endothelial cells (HRMECs) and human retinal pigment epithelial ARPE-19 cells. Bezafibrate (30-100 μM) suppresses tumor necrosis factor (TNF)α induced inflammatory factors and regulates TNFα induced nuclear factor (NF)-κB transactivation in HRMEC. Bezafibrate inhibits VEGF-induced HRMECs migration, and inhibits interleukin (IL)-1β-induced VEGF secretion of ARPE-19 cells[2].In Vivo:Bezafibrate (0.5%) markedly reduces plasma lipid and glucose levels, and increases islet area in the pancreas in TallyHo mice. Bezafibrate also improves energy expenditure and metabolic flexibility. Moreover, Bezafibrate ameliorates steatosis, modifies lipid composition and increases mitochondrial mass in the liver[3]. References: | |||||

Bezafibrate Dilution Calculator

Bezafibrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7638 mL | 13.819 mL | 27.6381 mL | 55.2761 mL | 69.0951 mL |
| 5 mM | 0.5528 mL | 2.7638 mL | 5.5276 mL | 11.0552 mL | 13.819 mL |
| 10 mM | 0.2764 mL | 1.3819 mL | 2.7638 mL | 5.5276 mL | 6.9095 mL |
| 50 mM | 0.0553 mL | 0.2764 mL | 0.5528 mL | 1.1055 mL | 1.3819 mL |
| 100 mM | 0.0276 mL | 0.1382 mL | 0.2764 mL | 0.5528 mL | 0.691 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bezafibrate is the first clinically tested dual and pan-PPAR co-agonism.
- Alatamine
Catalog No.:BCN3100
CAS No.:41855-33-8
- Mangiferolic acid
Catalog No.:BCN4636
CAS No.:4184-34-3
- Hesperetin-7-methyl ether
Catalog No.:BCN8501
CAS No.:
- Junicedric acid
Catalog No.:BCN7660
CAS No.:41787-69-3
- Hypecorinine
Catalog No.:BCN3298
CAS No.:41787-57-9
- H-Leu-pNA.HCl
Catalog No.:BCC2972
CAS No.:4178-93-2
- Lenvatinib (E7080)
Catalog No.:BCC1172
CAS No.:417716-92-8
- 14-Deoxyandrographolide
Catalog No.:BCN3706
CAS No.:4176-97-0
- ar-Curcumene
Catalog No.:BCN7534
CAS No.:4176-06-1
- Evonimine
Catalog No.:BCN3082
CAS No.:41758-69-4
- Dihydrophaseic acid
Catalog No.:BCN5478
CAS No.:41756-77-8
- Ophiopogonin D
Catalog No.:BCN5004
CAS No.:41753-55-3
- 2,3-DCPE hydrochloride
Catalog No.:BCC2384
CAS No.:418788-90-6
- PYR-41
Catalog No.:BCC4470
CAS No.:418805-02-4
- Nitrocefin
Catalog No.:BCC6544
CAS No.:41906-86-9
- Isopropyl 4-Hydroxybenzoate
Catalog No.:BCN8409
CAS No.:4191-73-5
- Trilobatin
Catalog No.:BCN5479
CAS No.:4192-90-9
- N1-Methyl-4-nitrobenzene-1,2-diamine
Catalog No.:BCC9070
CAS No.:41939-61-1
- MLR 1023
Catalog No.:BCC6232
CAS No.:41964-07-2
- Pinocembrin chalcone
Catalog No.:BCN7223
CAS No.:4197-97-1
- Glabranin
Catalog No.:BCN5480
CAS No.:41983-91-9
- (S)-(-)-Propranolol hydrochloride
Catalog No.:BCC6809
CAS No.:4199-10-4
- 5-Chloro-1,10-phenanthroline
Catalog No.:BCC3713
CAS No.:4199-89-7
- Dihydropashanone
Catalog No.:BCN4635
CAS No.:41997-41-5
Protective effects of bezafibrate against elaidic acid-induced accumulation of lipid droplets in monocytic cells.[Pubmed:28340693]
Curr Res Transl Med. 2017 Jan - Mar;65(1):20-30.
Some factors related to diet, such as trans fatty acids (TFA), are known to be involved in the progression of atherosclerosis in humans. Thus, the aim of our study was (i) to evaluate the effects of three dietary free fatty acids (FFA) (elaidic (EA), oleic (OA) and palmitic acid (PA)) on U937 human monocytes, and (ii) to study the eventual benefits of Bezafibrate (BZF), a pan-agonist for PPAR isoforms (alpha, gamma and delta) in U937 cells treated with FFA. Morphologic and functional changes were investigated by microscopic and flow cytometric methods. Cellular lipid content, lipid droplets and FA composition were identified and studied. All analyses were also realized in association with or without BZF. Contrary to OA and PA, EA slightly induced both propidium iodide-positive cells and mitochondrial depolarization. In addition, in contrast to OA and PA, EA induced only a slight increase in superoxide anion production. However, EA and OA promoted cytoplasmic lipid droplets accumulation. Only EA and OA significantly increased CD36 expression. It is noteworthy that BZF had a more or less pronounced protective effect against EA-, OA- and PA-induced side effects: BZF attenuated the impaired cell viability and inflammatory response, decreased superoxide anion production and prevented the accumulation of neutral and polar lipids. The effects were less pronounced with OA and PA than with EA. Altogether, our data revealed a benefit of BZF on the side effects induced especially with EA. It may thus be of interest in preventing the early stages of atherosclerotic plaque formation.
The PPAR pan-agonist bezafibrate ameliorates cardiomyopathy in a mouse model of Barth syndrome.[Pubmed:28279226]
Orphanet J Rare Dis. 2017 Mar 9;12(1):49.
BACKGROUND: The PGC-1alpha/PPAR axis has been proposed as a potential therapeutic target for several metabolic disorders. The aim was to evaluate the efficacy of the pan-PPAR agonist, Bezafibrate, in tafazzin knockdown mice (TazKD), a mouse model of Barth syndrome that exhibits age-dependent dilated cardiomyopathy with left ventricular (LV) dysfunction. RESULTS: The effect of Bezafibrate on cardiac function was evaluated by echocardiography in TazKD mice with or without beta-adrenergic stress. Adrenergic stress by chronic isoproterenol infusion exacerbates the cardiac phenotype in TazKD mice, significantly depressing LV systolic function by 4.5 months of age. Bezafibrate intake over 2 months substantially ameliorates the development of LV systolic dysfunction in isoproterenol-stressed TazKD mice. Without beta-adrenergic stress, TazKD mice develop dilated cardiomyopathy by 7 months of age. Prolonged treatment with suprapharmacological dose of Bezafibrate (0.5% in rodent diet) over a 4-month period effectively prevented LV dilation in mice isoproterenol treatment. Bezafibrate increased mitochondrial biogenesis, however also promoted oxidative stress in cardiomyocytes. Surprisingly, improvement of systolic function in Bezafibrate-treated mice was accompanied with simultaneous reduction of cardiolipin content and increase of monolysocardiolipin levels in cardiac muscle. CONCLUSIONS: Thus, we demonstrate that Bezafibrate has a potent therapeutic effect on preventing cardiac dysfunction in a mouse model of Barth syndrome with obvious implications for treating the human disease. Additional studies are needed to assess the potential benefits of PPAR agonists in humans with Barth syndrome.
Bezafibrate ameliorates diabetes via reduced steatosis and improved hepatic insulin sensitivity in diabetic TallyHo mice.[Pubmed:28271032]
Mol Metab. 2017 Jan 6;6(3):256-266.
OBJECTIVE: Recently, we have shown that Bezafibrate (BEZ), the pan-PPAR (peroxisome proliferator-activated receptor) activator, ameliorated diabetes in insulin deficient streptozotocin treated diabetic mice. In order to study whether BEZ can also improve glucose metabolism in a mouse model for fatty liver and type 2 diabetes, the drug was applied to TallyHo mice. METHODS: TallyHo mice were divided into an early (ED) and late (LD) diabetes progression group and both groups were treated with 0.5% BEZ (BEZ group) or standard diet (SD group) for 8 weeks. We analyzed plasma parameters, pancreatic beta-cell morphology, and mass as well as glucose metabolism of the BEZ-treated and control mice. Furthermore, liver fat content and composition as well as hepatic gluconeogenesis and mitochondrial mass were determined. RESULTS: Plasma lipid and glucose levels were markedly reduced upon BEZ treatment, which was accompanied by elevated insulin sensitivity index as well as glucose tolerance, respectively. BEZ increased islet area in the pancreas. Furthermore, BEZ treatment improved energy expenditure and metabolic flexibility. In the liver, BEZ ameliorated steatosis, modified lipid composition and increased mitochondrial mass, which was accompanied by reduced hepatic gluconeogenesis. CONCLUSIONS: Our data showed that BEZ ameliorates diabetes probably via reduced steatosis, enhanced hepatic mitochondrial mass, improved metabolic flexibility and elevated hepatic insulin sensitivity in TallyHo mice, suggesting that BEZ treatment could be beneficial for patients with NAFLD and impaired glucose metabolism.
Bezafibrate Attenuates Pressure Overload-Induced Cardiac Hypertrophy and Fibrosis.[Pubmed:28127304]
PPAR Res. 2017;2017:5789714.
Background. Peroxisome proliferator-activated receptor-alpha (PPAR-alpha) is closely associated with the development of cardiac hypertrophy. Previous studies have indicated that Bezafibrate (BZA), a PPAR-alpha agonist, could attenuate insulin resistance and obesity. This study was designed to determine whether BZA could protect against pressure overload-induced cardiac hypertrophy. Methods. Mice were orally given BZA (100 mg/kg) for 7 weeks beginning 1 week after aortic banding (AB) surgery. Cardiac hypertrophy was assessed based on echocardiographic, histological, and molecular aspects. Moreover, neonatal rat ventricular cardiomyocytes (NRVMs) were used to investigate the effects of BZA on the cardiomyocyte hypertrophic response in vitro. Results. Our study demonstrated that BZA could alleviate cardiac hypertrophy and fibrosis in mice subjected to AB surgery. BZA treatment also reduced the phosphorylation of protein kinase B (AKT)/glycogen synthase kinase-3beta (GSK3beta) and mitogen-activated protein kinases (MAPKs). BZA suppressed phenylephrine- (PE-) induced hypertrophy of cardiomyocyte in vitro. The protective effects of BZA were abolished by the treatment of the PPAR-alpha antagonist in vitro. Conclusions. BZA could attenuate pressure overload-induced cardiac hypertrophy and fibrosis.


