Nociceptin (1-13)NH2Potent ORL1 agonist CAS# 178064-02-3 |
- Mibefradil
Catalog No.:BCC1748
CAS No.:116644-53-2
- Mibefradil dihydrochloride
Catalog No.:BCC1749
CAS No.:116666-63-8
- Cilnidipine
Catalog No.:BCC1083
CAS No.:132203-70-4
- Pregabalin
Catalog No.:BCN2175
CAS No.:148553-50-8
- NP118809
Catalog No.:BCC1807
CAS No.:41332-24-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 178064-02-3 | SDF | Download SDF |
| PubChem ID | 6324645 | Appearance | Powder |
| Formula | C61H100N22O15 | M.Wt | 1381.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 0.67 mg/ml in 20% acetonitrile | ||
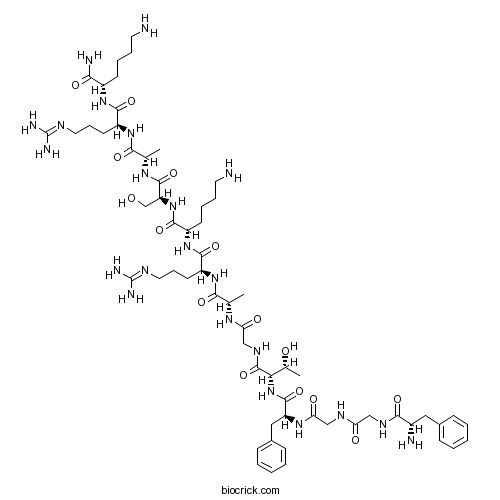
| Sequence | FGGFTGARKSARK (Modifications: Lys-13 = C-terminal amide) | ||
| Chemical Name | (2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S,3R)-2-[[(2S)-2-[[2-[[2-[[(2S)-2-amino-3-phenylpropanoyl]amino]acetyl]amino]acetyl]amino]-3-phenylpropanoyl]amino]-3-hydroxybutanoyl]amino]acetyl]amino]propanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]hexanoyl]amino]-3-hydroxypropanoyl]amino]propanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]hexanamide | ||
| SMILES | CC(C(C(=O)NCC(=O)NC(C)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCCN)C(=O)NC(CO)C(=O)NC(C)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCCN)C(=O)N)NC(=O)C(CC1=CC=CC=C1)NC(=O)CNC(=O)CNC(=O)C(CC2=CC=CC=C2)N)O | ||
| Standard InChIKey | RHMALYOXPBRJBG-CGUXNFSNSA-N | ||
| Standard InChI | InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71)/t34-,35-,36+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bioactive metabolite of nociceptin and potent agonist for the ORL1 receptor (pEC50 = 7.9 in mouse vas deferens, Ki = 0.75 nM for binding to rat forebrain membranes). |

Nociceptin (1-13)NH2 Dilution Calculator

Nociceptin (1-13)NH2 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Aescigenin
Catalog No.:BCC8293
CAS No.:17806-68-7
- Fmoc-D-Phe(4-NO2)-OH
Catalog No.:BCC3278
CAS No.:177966-63-1
- Allopurinol Sodium
Catalog No.:BCC4886
CAS No.:17795-21-0
- Sauchinone
Catalog No.:BCN2299
CAS No.:177931-17-8
- Clematichinenoside C
Catalog No.:BCN7850
CAS No.:177912-24-2
- Boc-His-OH
Catalog No.:BCC3398
CAS No.:17791-52-5
- Eletriptan HBr
Catalog No.:BCC5039
CAS No.:177834-92-3
- Calystegine A6
Catalog No.:BCN1886
CAS No.:177794-04-6
- Calystegine N1
Catalog No.:BCN1866
CAS No.:177794-03-5
- Proxyfan oxalate
Catalog No.:BCC7378
CAS No.:177708-09-7
- NKP608
Catalog No.:BCC1802
CAS No.:177707-12-9
- MNITMT
Catalog No.:BCC7382
CAS No.:177653-76-8
- Bacopasaponin C
Catalog No.:BCC8124
CAS No.:178064-13-6
- 3-Deoxyzinnolide
Catalog No.:BCN4799
CAS No.:17811-32-4
- H-Asp-OMe
Catalog No.:BCC2884
CAS No.:17812-32-7
- 6,7,4'-Trihydroxyisoflavone
Catalog No.:BCN2910
CAS No.:17817-31-1
- Hardwickiic acid
Catalog No.:BCN1132
CAS No.:1782-65-6
- Linderone
Catalog No.:BCN1133
CAS No.:1782-79-2
- Tetrahymanone
Catalog No.:BCN6932
CAS No.:17822-06-9
- Calystegine B3
Catalog No.:BCN1880
CAS No.:178231-95-3
- Orphanin FQ (1-11)
Catalog No.:BCC6085
CAS No.:178249-41-7
- Nociceptin (1-7)
Catalog No.:BCC5738
CAS No.:178249-42-8
- H-D-Asp-OH
Catalog No.:BCC2894
CAS No.:1783-96-6
- 12-Hydroxy-6-epi-albrassitriol
Catalog No.:BCN7460
CAS No.:178330-78-4
Structure activity studies of nociceptin/orphanin FQ(1-13)-NH2 derivatives modified in position 5.[Pubmed:25716007]
Bioorg Med Chem. 2015 Apr 1;23(7):1515-20.
Nociceptin/orphanin FQ (N/OFQ) is a heptadecapeptide acting as the endogenous ligand of the N/OFQ peptide receptor (NOP). N/OFQ(1-13)-NH2 is the shortest N/OFQ sequence maintaining the same potency and efficacy as the natural peptide. Thus N/OFQ(1-13)-NH2 was used as chemical template for investigating the structure activity relationship of threonine in position 5. 28 [X(5)]N/OFQ(1-13)-NH2 derivatives, in which Thr was substituted with natural and unnatural residues, were synthesized and characterized pharmacologically for their effects at the human NOP receptor. Two different functional assays were used: agonist stimulated [(35)S]GTPgammaS binding in cell membranes and calcium mobilization in whole cells co-expressing chimeric G proteins. All [X(5)]N/OFQ(1-13)-NH2 derivatives behaved as full NOP agonists showing large differences in their potency. There was an excellent correlation between the results obtained in the two assays. The results of this study suggest that: position 5 does not play a pivotal role in receptor activation; the secondary alcoholic function of Thr is not important for receptor binding; side chain size, lipo/hydrophilic balance as well as hydrogen bond capability are also not crucial for receptor binding; an aliphatic amino function positively charged with at least 3 carbon atom distance from the peptide backbone has a huge disrupting effect on receptor binding. In conclusion this study demonstrates that a simple ethyl side chain as in compound 23 is sufficient in N/OFQ position 5 for maintaining bioactivity.
[Dmt1]N/OFQ(1-13)-NH2: a potent nociceptin/orphanin FQ and opioid receptor universal agonist.[Pubmed:22827708]
Br J Pharmacol. 2013 Jan;168(1):151-62.
BACKGROUND AND PURPOSE: Intrathecally (i.t.) administered nociceptin/orphanin FQ (N/OFQ) evokes antinociceptive effects in rodents. Recent studies in monkeys demonstrated that i.t. co-application of N/OFQ and morphine elicits synergistic antinociceptive actions suggesting mixed N/OFQ peptide (NOP) and mu opioid receptor agonists as innovative spinal analgesics. Thus, novel N/OFQ related peptides were synthesized in order to identify and pharmacologically characterize a mixed NOP/ mu opioid receptor agonist. EXPERIMENTAL APPROACH: The following in vitro assays were used: calcium mobilization in cells expressing the human NOP or classical opioid receptors and chimeric G proteins, receptor and [(35)S]-GTPgammaS binding, [(35)S]-GTPgammaS binding in rat spinal cord membranes, guinea pig ileum bioassay. In vivo experiments were performed in monkeys using the tail withdrawal assay. KEY RESULTS: From calcium mobilization studies [Dmt(1)]N/OFQ(1-13)-NH(2) was selected as the most potent and least selective compound. The mixed NOP/opioid full agonist activity and high affinity of [Dmt(1)]N/OFQ(1-13)-NH(2) was confirmed at human recombinant receptors in receptor binding, calcium mobilization and/or [(35)S]-GTPgammaS binding studies, at rat spinal cord receptors in [(35)S]-GTPgammaS binding experiments, and at guinea pig receptors inhibiting neurogenic contractions in the ileum. In vivo in the tail withdrawal assay in monkeys i.t. [Dmt(1) ]N/OFQ(1-13)-NH(2) was able to elicit robust and long-lasting antinociceptive effects. CONCLUSIONS AND IMPLICATIONS: Collectively, these results demonstrate that [Dmt(1)]N/OFQ(1-13)-NH(2) behaves as NOP/opioid receptor universal agonist and substantiate the suggestion that such mixed ligands are worthy of development as innovative spinal analgesics.
Role of nociceptin/orphanin FQ and the pseudopeptide [Phe1Psi(CH2NH)Gly2]-nociceptin(1-13)-NH2 and their interaction with classic opioids in the modulation of thermonociception in the land snail Helix aspersa.[Pubmed:18096155]
Eur J Pharmacol. 2008 Feb 26;581(1-2):77-85.
The role in nociception of nociceptin/orphanin FQ (N/OFQ) and its receptor, the opioid receptor-like 1 (NOP), remains unclear because this peptide has been implicated in both suppression and enhancement of nociception. The present work characterises the effects of N/OFQ and the NOP receptor antagonist, the pseudopeptide [Phe(1)Psi(CH(2)NH)Gly(2)]-nociceptin(1-13)-NH(2) (Phe(1)Psi), on thermonociception in the snail Helix aspersa using the hot plate assay. Additionally, the possible interaction of each of these compounds with morphine or dynorphin A(1-17) and naloxone was studied. Compounds were administered into the hemocoel cavity of H. aspersa and the latency to the aversive withdrawal behaviour recorded. Dose-response and time course curves were done. N/OFQ and naloxone produced a similar dose-dependent pronociceptive effect; however, N/OFQ reached its peak effect earlier and was 30 times more potent than naloxone. [Phe(1)Psi(CH(2)NH)Gly(2)]-nociceptin(1-13)-NH(2) and the opioid agonists, morphine and dynorphin A(1-17) produced antinociception with a similar efficacy, but [Phe(1)Psi(CH(2)NH)Gly(2)]-nociceptin(1-13)-NH(2) reached its peak effect more rapidly and lasted longer than that of dynorphin A(1-17) and morphine. [Phe(1)Psi(CH(2)NH)Gly(2)]-nociceptin(1-13)-NH(2) was 50 times less potent than dynorphin A(1-17), but 30 times more potent than morphine. N/OFQ significantly reduced morphine and dynorphin A(1-17)-induced antinociception. Combined administration of low doses of [Phe(1)Psi(CH(2)NH)Gly(2)]-nociceptin(1-13)-NH(2) and morphine or dynorphin A(1-17) produced a potent antinociceptive effect. Sub-effective doses of naloxone and N/OFQ also synergised to produce pronociception. Data suggest that these two opioid classes regulate nociception through parallel systems. The H. aspersa model appears as a valuable experimental preparation to continue the study of these opioid receptor systems.
Are nociceptin(1-13)NH2 and its structural analogue [ORN(9)]nociceptin(1-13)NH2 able to affect brain antioxidant status in control and kainic acid-treated rats?[Pubmed:19418488]
Cell Biochem Funct. 2009 Jun;27(4):243-50.
In-vivo effects of nociceptin (N/OFQ(1-13)NH(2)) on the levels of lipid peroxidation and cell enzyme (superoxide dismutase, glutathione peroxidase and glutathione reductase) and non-enzyme (glutathione) antioxidants in brain of control and kainic acid-treated rats were studied. N/OFQ(1-13)NH(2) effects were compared with those of its structural analogue [Orn(9)]N/OFQ(1-13)NH(2). Kainic acid (25 microg, i.c.v) increased the lipid peroxidation (4 and 24 h after kainic acid treatment) and decreased the glutathione level (1 h after kainic acid injection). We failed to find, any changes in antioxidant enzyme activities, independently of the time of kainic acid treatment. At the background of kainic acid-effects, N/OFQ(1-13)NH(2) and [Orn(9)] N/OFQ(1-13)NH(2), injected 30 min before kainic acid, had no effects on all parameters, tested in brain. In addition, the neuropeptides did not change the antioxidant status in brain of control animals. It might be concluded that N/OFQ(1-13)NH(2) and [Orn(9)]N/OFQ(1-13)NH(2) have neither pro- nor anti-oxidant activity.
Pharmacology of nociceptin and its receptor: a novel therapeutic target.[Pubmed:10742280]
Br J Pharmacol. 2000 Apr;129(7):1261-83.
Nociceptin (NC), alias Orphanin FQ, has been recently identified as the endogenous ligand of the opioid receptor-like 1 receptor (OP(4)). This new NC/OP(4) receptor system belongs to the opioid family and has been characterized pharmacologically with functional and binding assays on native (mouse, rat, guinea-pig) and recombinant (human) receptors, by using specific and selective agonists (NC, NC(1 - 13)NH(2)) and a pure and competitive antagonist, [Nphe(1)]NC(1 - 13)NH(2). The similar order of potency of agonists and affinity values of the antagonist indicate that the same receptor is present in the four species. OP(4) is expressed in neurons, where it reduces activation of adenylyl cyclase and Ca(2+) channels while activating K(+) channels in a manner similar to opioids. In this way, OP(4) mediates inhibitory effects in the autonomic nervous system, but its activities in the central nervous system can be either similar or opposite to those of opioids. In vivo experiments have demonstrated that NC modulates a variety of biological functions ranging from nociception to food intake, from memory processes to cardiovascular and renal functions, from spontaneous locomotor activity to gastrointestinal motility, from anxiety to the control of neurotransmitter release at peripheral and central sites. These actions have been demonstrated using NC and various pharmacological tools, as antisense oligonucleotides targeting OP(4) or the peptide precursor genes, antibodies against NC, an OP(4) receptor selective antagonist and with data obtained from animals in which the receptor or the peptide precursor genes were knocked out. These new advances have contributed to better understanding of the pathophysiological role of the NC/OP(4) system, and ultimately will help to identify the therapeutic potential of new OP(4) receptor ligands.
Nociceptin receptor binding in mouse forebrain membranes: thermodynamic characteristics and structure activity relationships.[Pubmed:9884077]
Br J Pharmacol. 1998 Dec;125(7):1485-90.
The present study describes the labelling of the nociceptin (NC) receptor, ORL1, in mouse forebrain membranes with a new ligand partially protected from metabolic degradation at the C-terminal; the ligand, [3H]-NC-NH2, has a specific activity of 24.5 Ci mmol(-1). Saturation experiments revealed a single class of binding sites with a KD value of 0.55 nM and Bmax of 94 fmol mg(-1) of protein. Non specific binding was 30% of total binding. Kinetic binding studies yielded the following rate constants: Kobs = 0.104 min(-1); K1 =0.034 min(-1): T1/2=20 min; K(+1)=0.07 min nM(-1). Thermodynamic analyses indicated that [3H]-NC-NH2 binding to the mouse ORL1 is totally entropy driven, similar to what has been observed for the labelled agonists to the opioid receptors OP1(delta), OP2(kappa) and OP3(mu). Receptor affinities of several NC fragments and analogues, including the newly discovered ORL-1 receptor antagonist [Phe1psi(CH2-NH)Gly2]NC(1-13)-NH2([F/G]NC(1-13)-NH2), were also evaluated in displacement experiments. The competition curves for these compounds were found to be parallel to that of NC and the following order of potency was determined for NC fragments: NC-OH = NC-NH2-NC(1-13)-NH2 > > NC(1-12)-NH2 > NC(1-13)-OH > > NC(1-11)-NH2, and for NC and NC(1-13)-NH2 analogues: [Tyr1]NC-NH2 > or = [Leu1]NC(1-13)-NH2 > or = [Tyr1]NC(1-13)-NH2 > or = [F/G]NC(1-13)-NH2 > > [Phe3]NC(1-13)-NH2 > [DF/G]NC(1-13)-NH2. Standard opioid receptor ligands (either agonists or antagonists) were unable to displace [3H]-NC-NH2 binding when applied at concentrations up to 10 microM indicating that this new radioligand interacts with a non opioid site, probably the ORL1 receptor.


