Allopurinol SodiumCAS# 17795-21-0 |
- LDK378
Catalog No.:BCC3691
CAS No.:1032900-25-6
- LDN193189 Hydrochloride
Catalog No.:BCC1695
CAS No.:1062368-62-0
- AP26113
Catalog No.:BCC1069
CAS No.:1197958-12-5
- SB525334
Catalog No.:BCC2531
CAS No.:356559-20-1
- TAE684 (NVP-TAE684)
Catalog No.:BCC3660
CAS No.:761439-42-3
- (R)-Crizotinib
Catalog No.:BCC1284
CAS No.:877399-52-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 17795-21-0 | SDF | Download SDF |
| PubChem ID | 23662349 | Appearance | Powder |
| Formula | C5H4N4NaO | M.Wt | 159.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
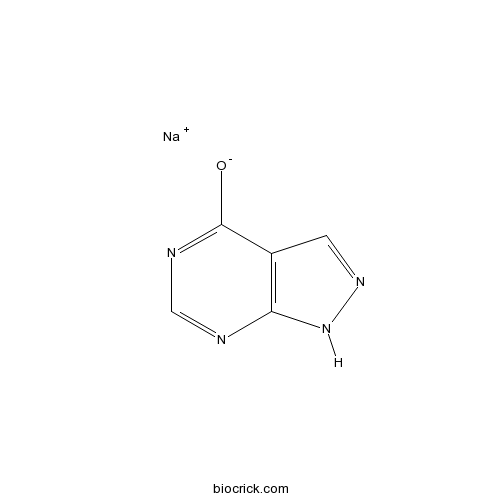
| Chemical Name | sodium;1H-pyrazolo[3,4-d]pyrimidin-4-olate | ||
| SMILES | [Na+].[O-]c1ncnc2[nH]ncc12 | ||
| Standard InChIKey | PTJRZVJXXNYNLN-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C5H4N4O.Na/c10-5-3-1-8-9-4(3)6-2-7-5;/h1-2H,(H2,6,7,8,9,10);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Allopurinol Sodium Dilution Calculator

Allopurinol Sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.2854 mL | 31.4268 mL | 62.8536 mL | 125.7071 mL | 157.1339 mL |
| 5 mM | 1.2571 mL | 6.2854 mL | 12.5707 mL | 25.1414 mL | 31.4268 mL |
| 10 mM | 0.6285 mL | 3.1427 mL | 6.2854 mL | 12.5707 mL | 15.7134 mL |
| 50 mM | 0.1257 mL | 0.6285 mL | 1.2571 mL | 2.5141 mL | 3.1427 mL |
| 100 mM | 0.0629 mL | 0.3143 mL | 0.6285 mL | 1.2571 mL | 1.5713 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Allopurinol Sodium
- Sauchinone
Catalog No.:BCN2299
CAS No.:177931-17-8
- Clematichinenoside C
Catalog No.:BCN7850
CAS No.:177912-24-2
- Boc-His-OH
Catalog No.:BCC3398
CAS No.:17791-52-5
- Eletriptan HBr
Catalog No.:BCC5039
CAS No.:177834-92-3
- Calystegine A6
Catalog No.:BCN1886
CAS No.:177794-04-6
- Calystegine N1
Catalog No.:BCN1866
CAS No.:177794-03-5
- Proxyfan oxalate
Catalog No.:BCC7378
CAS No.:177708-09-7
- NKP608
Catalog No.:BCC1802
CAS No.:177707-12-9
- MNITMT
Catalog No.:BCC7382
CAS No.:177653-76-8
- Glycerol 1-(26-hydroxyhexacosanoate)
Catalog No.:BCN1131
CAS No.:177602-14-1
- ZK 164015
Catalog No.:BCC7272
CAS No.:177583-70-9
- Flavokawain B
Catalog No.:BCN3568
CAS No.:1775-97-9
- Fmoc-D-Phe(4-NO2)-OH
Catalog No.:BCC3278
CAS No.:177966-63-1
- Aescigenin
Catalog No.:BCC8293
CAS No.:17806-68-7
- Nociceptin (1-13)NH2
Catalog No.:BCC5749
CAS No.:178064-02-3
- Bacopasaponin C
Catalog No.:BCC8124
CAS No.:178064-13-6
- 3-Deoxyzinnolide
Catalog No.:BCN4799
CAS No.:17811-32-4
- H-Asp-OMe
Catalog No.:BCC2884
CAS No.:17812-32-7
- 6,7,4'-Trihydroxyisoflavone
Catalog No.:BCN2910
CAS No.:17817-31-1
- Hardwickiic acid
Catalog No.:BCN1132
CAS No.:1782-65-6
- Linderone
Catalog No.:BCN1133
CAS No.:1782-79-2
- Tetrahymanone
Catalog No.:BCN6932
CAS No.:17822-06-9
- Calystegine B3
Catalog No.:BCN1880
CAS No.:178231-95-3
- Orphanin FQ (1-11)
Catalog No.:BCC6085
CAS No.:178249-41-7
Pulmonary vasodilator responses to sodium nitrite are mediated by an allopurinol-sensitive mechanism in the rat.[Pubmed:19074675]
Am J Physiol Heart Circ Physiol. 2009 Feb;296(2):H524-33.
Recent studies show that pulmonary vasodilator responses to nitrite are enhanced by hypoxia. However, the mechanism by which nitrite is converted to vasoactive nitric oxide (NO) is uncertain. In the present study, intravenous injections of sodium nitrite decreased pulmonary and systemic arterial pressures and increased cardiac output. The decreases in pulmonary arterial pressure were enhanced when tone in the pulmonary vascular bed was increased with U-46619. Under elevated tone conditions, decreases in pulmonary and systemic arterial pressures in response to nitrite were attenuated by allopurinol in a dose that did not alter responses to the NO donors, sodium nitroprusside and diethylamine/NO, suggesting that xanthine oxidoreductase is the major enzyme-reducing nitrite to NO. Ventilation with a 10% O(2) gas mixture increased pulmonary arterial pressure, and the response to hypoxia was enhanced by N(G)-nitro-l-arginine methyl ester and not altered by allopurinol. This suggests that NO formed by the endothelium and not from the reduction of plasma nitrite modulates the hypoxic pulmonary vasoconstrictor response. Although intravenous injections of sodium nitrite reversed pulmonary hypertensive responses to U-46619, hypoxia, and N(G)-nitro-l-arginine methyl ester, the pulmonary vasodilator response to nitrite was not altered by ventilation with 10% O(2) when baseline pulmonary arterial pressure was increased to similar values in animals breathing room air or the hypoxic gas. These data provide evidence that xanthine oxidoreductase is the major enzyme-reducing nitrite to vasoactive NO, and that this mechanism is not modified by hypoxia.
Allopurinol and enalapril failed to conserve urinary NOx and sodium in ischemic acute renal failure in spontaneously hypertensive rats.[Pubmed:16900002]
Am J Nephrol. 2006;26(4):388-99.
BACKGROUND: Ischemia-reperfusion-induced acute renal failure (ARF) is associated with a high mortality in patients with hypertension and with an unfavorable outcome of kidney transplants from marginal donors. AIM: The influence of allopurinol and enalapril on urinary nitrate/nitrite (UNOx), glomerular filtration rate, plasma and urinary sodium, and hemodynamic parameters was examined in spontaneously hypertensive rats (SHR) with ARF. METHODS: ARF was induced by right-kidney removal and clamping the left renal artery for 40 min in 50 male 26-week-old SHR weighing 300 +/- 23 g. The rats were randomly allocated to five groups: (1) sham operated; (2) ARF; (3) ARF after pretreatment with 40 mg/kg allopurinol; (4) ARF after pretreatment with 40 mg/kg enalapril, and (5) ARF after pretreatment with 40 mg/kg allopurinol and 40 mg/kg enalapril. Creatinine clearance, UNOx (Griess reaction), cardiac output (dye dilution technique), mean arterial blood pressure, and renal blood flow were measured 24 h after reperfusion. Total vascular resistance and renal vascular resistance were calculated and compared between the groups. RESULTS: A nonsignificant decrease was found in both daily UNOx excretion and creatinine clearance when pretreated ARF groups and the ARF group without pretreatment were compared (p > 0.05). Significantly lower plasma sodium values (139.5 +/- 4.86 mmol/l) in the allopurinol-pretreated ARF group were found than in the ARF group without pretreatment, in the ARF group pretreated with enalapril, and in the sham SHR group (p = 0.029). The urinary sodium loss was greater in the enalapril-pretreated than in the allopurinol-pretreated ARF group (p = 0.047). Allopurinol and/or enalapril pretreatment decreased total vascular resistance (p = 0.003) in comparison with the sham SHR group. CONCLUSION: Neither allopurinol nor enalapril nor both were protective against ischemia-reperfusion injury in SHR, nor altered glomerular filtration rate and UNOx in a favorable direction.
Clinical and serological follow-up in dogs with visceral leishmaniosis treated with allopurinol and sodium stibogluconate.[Pubmed:15740861]
Vet Parasitol. 2005 Mar 31;128(3-4):243-9.
Seven dogs with parasitologically proven clinical visceral leishmaniosis (Leishmania infantum infection) were treated with a combination of allopurinol and sodium stibogluconate. The dogs received first orally 15 mg/kg of allopurinol every 12 h until the clinical signs improved, in the following 1 month period allopurinol at same dose and subcutaneously 30 mg/kg of sodium stibogluconate combination were given daily and at the end of the combined treatment, allopurinol was continued alone at the same dose till the end of 8 months. During the treatment period, dogs were supported by additional proteins, vitamins, and minerals. A long acting insecticide (collar or drop) was also used in order to prevent further parasite transmission. Follow-up was maintained by clinical, clinicopathological evaluation, and parasitological examination of lymph node, serology using the indirect immunofluorescent antibody test (IFAT). Before treatment commenced, the most important clinical signs were exfoliative dermatitis, ulcerations, peripheral lymhadenopathy, pale mucous membranes, weight loss, and ocular lesions. Clinicopathological findings included commonly anaemia, hyperproteinaemia, hyperglobulinaemia and hypoalbuminaemia. Before the treatment, amastigotes were seen in six of the seven dogs by examination of lymph node aspiration, and IFAT-titers were positive in all dogs. At the end of 8 months treatment, remission of clinical signs, restoration to normal of clinicopathological abnormalities were noticed. Lymph node aspiration was performed on three out of the seven dogs at the end of the treatment because of the very small sizes of the lymph nodes, and no amastigotes were observed. Although the mean IFAT-titer of the dogs were significantly (P < 0.001) lower compared with pretreatment, IFAT-titers of dogs were still positive. No relapses occurred during treatment period and a 6-24-month duration after the end of therapy. Based on the above results, long-term use of allopurinol combined with sodium stibogluconate together with support treatment concluded to have enough therapeutic efficacies in the treatment of dogs with visceral leishmaniosis. Observations of the cases for possible relapses were still going on and insecticide application was carefully carrying on in order preventing a possible re-infection.
A retrospective study of intravenous sodium stibogluconate alone and in combinations with allopurinol, rifampicin, and an immunomodulator in the treatment of Indian post-kala-azar dermal leishmaniasis.[Pubmed:20228542]
Indian J Dermatol Venereol Leprol. 2010 Mar-Apr;76(2):138-44.
BACKGROUND AND AIMS: A retrospective analysis of treatment outcome using recommended dose of sodium stibogluconate (SSG) alone and in combination with other antileishmanial drugs in adults with post-kala-azar dermal leishmaniasis (PKDL) attending as outpatients. METHODS: A total of 61 patients seen over ten years were included in the report. All had polymorphic lesions. Diagnosis was based on clinical picture, hailing from kala-azar (KA) endemic area, exclusion of other dermatoses, histopathology, and therapeutic response. Patients were distributed into two groups: Group I (n = 32), where SSG was given intravenously; in Group II (n = 29), they were allocated to one of four categories using SSG in combination with other drugs. In the first category, SSG was given along with allopurinol (n = 10); in second with rifampicin (n = 6); and in third with both allopurinol and rifampicin (n = 5). In the fourth category, SSG was administered with an immunomodulator (n = 8), Mw vaccine, known to enhance host Th1 response. RESULTS: Only 12 out of 61 patients completed treatment till histopathologic evidence of cure, five in Group I and seven in Group II, no patient being from third category. None had taken SSG without interruptions. Time taken for papulonodules to subside was similar in both groups, but erythema and induration subsided earlier in Group II. Group I patients attained cure after 120 injections while in Group II it took 95 injections in SSG + allopurinol and Mw vaccine categories respectively, and 110 with SSG + rifampicin. Nevertheless this was insufficient to facilitate compliance. Poor performance and high dropouts related to long duration of therapy, thrombophlebitis, difficulty in accessing veins, disabling rheumatic side-effects and practical problems. Liver, renal and pancreatic functions and ECG remained normal. CONCLUSION: No major advantage was obtained using allopurinol, rifampicin or Mw vaccine along with SSG as compared to SSG alone.


