PindololCAS# 13523-86-9 |
- GSK256066 2,2,2-trifluoroacetic acid
Catalog No.:BCC1605
CAS No.:1415560-64-3
- Nortadalafil
Catalog No.:BCC1806
CAS No.:171596-36-4
- Bay 60-7550
Catalog No.:BCC1405
CAS No.:439083-90-6
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
- AN-2728
Catalog No.:BCC1361
CAS No.:906673-24-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13523-86-9 | SDF | Download SDF |
| PubChem ID | 4828 | Appearance | Powder |
| Formula | C14H20N2O2 | M.Wt | 248.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (201.35 mM; Need ultrasonic) | ||
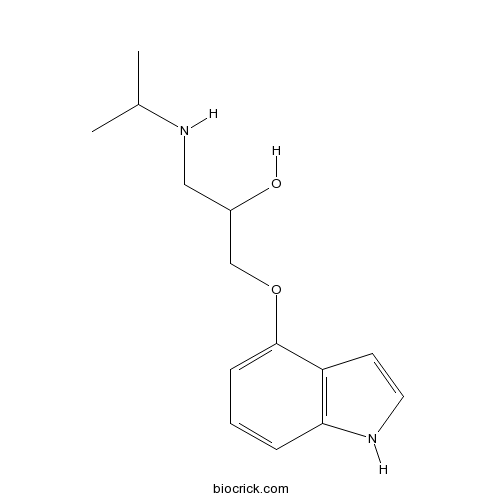
| Chemical Name | 1-(1H-indol-4-yloxy)-3-(propan-2-ylamino)propan-2-ol | ||
| SMILES | CC(C)NCC(COC1=CC=CC2=C1C=CN2)O | ||
| Standard InChIKey | JZQKKSLKJUAGIC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H20N2O2/c1-10(2)16-8-11(17)9-18-14-5-3-4-13-12(14)6-7-15-13/h3-7,10-11,15-17H,8-9H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-HT1A/1B receptor antagonist, with roughly equal affinity for each subtype. A partial agonist at mouse and human β3-adrenoceptors. (S)-(-)-Pindolol also available. |

Pindolol Dilution Calculator

Pindolol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0271 mL | 20.1353 mL | 40.2706 mL | 80.5412 mL | 100.6765 mL |
| 5 mM | 0.8054 mL | 4.0271 mL | 8.0541 mL | 16.1082 mL | 20.1353 mL |
| 10 mM | 0.4027 mL | 2.0135 mL | 4.0271 mL | 8.0541 mL | 10.0677 mL |
| 50 mM | 0.0805 mL | 0.4027 mL | 0.8054 mL | 1.6108 mL | 2.0135 mL |
| 100 mM | 0.0403 mL | 0.2014 mL | 0.4027 mL | 0.8054 mL | 1.0068 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AZD6738
Catalog No.:BCC6505
CAS No.:1352226-88-0
- Schineolignin B
Catalog No.:BCN3623
CAS No.:1352185-26-2
- AMG232
Catalog No.:BCC3992
CAS No.:1352066-68-2
- 3Beta-acetoxy-eupha-7,25-dien-24(R)-ol
Catalog No.:BCN1580
CAS No.:1352001-09-2
- Sanggenol P
Catalog No.:BCN4766
CAS No.:1351931-30-0
- H-Val-OtBu.HCl
Catalog No.:BCC3143
CAS No.:13518-40-6
- GNE-7915
Catalog No.:BCC5304
CAS No.:1351761-44-8
- HG-10-102-01
Catalog No.:BCC4271
CAS No.:1351758-81-0
- ONO-4059
Catalog No.:BCC6463
CAS No.:1351635-67-0
- 21,23:24,25-Diepoxy-21,23-dimethoxytirucall-7-en-3-one
Catalog No.:BCN1581
CAS No.:1351617-74-7
- Amooracetal
Catalog No.:BCN6876
CAS No.:1351617-73-6
- Sarpogrelate hydrochloride
Catalog No.:BCC5247
CAS No.:135159-51-2
- Trijuganone C
Catalog No.:BCN3685
CAS No.:135247-94-8
- Pulchinenoside B
Catalog No.:BCN6554
CAS No.:135247-95-9
- EW-7197
Catalog No.:BCC6467
CAS No.:1352608-82-2
- 2''-O-Rhamnosylicariside II
Catalog No.:BCN3464
CAS No.:135293-13-9
- MEN 10376
Catalog No.:BCC7133
CAS No.:135306-85-3
- 8,8'-Bibaicalein
Catalog No.:BCN6549
CAS No.:135309-02-3
- 5,7,3',4'-Tetrahydroxy-3-methoxy-8,5'-diprenylflavone
Catalog No.:BCN6848
CAS No.:1353676-65-9
- Isojasminin
Catalog No.:BCN7492
CAS No.:135378-08-4
- 4'-Hydroxyisojasminin
Catalog No.:BCN7383
CAS No.:135378-09-5
- 12alpha-Methoxygrandiflorenic acid
Catalog No.:BCN7771
CAS No.:135383-94-7
- 8-Prenyldaidzein
Catalog No.:BCN4711
CAS No.:135384-00-8
- CX-6258 hydrochloride hydrate
Catalog No.:BCC1505
CAS No.:1353858-99-7
Is pindolol augmentation effective in depressed patients resistant to selective serotonin reuptake inhibitors? A systematic review and meta-analysis.[Pubmed:25689398]
Hum Psychopharmacol. 2015 May;30(3):132-42.
OBJECTIVE: This systematic review and meta-analysis was conducted to assess the use of Pindolol augmentation in depressed patients resistant to selective serotonin reuptake inhibitor (SSRI) therapy. METHODS: A comprehensive search of PubMed, Cochrane, Embase, Web of Science, and PsychINFO databases from 1970 through December 2013 was conducted. Only randomized controlled trials (RCTs) studied on unipolar SSRI-resistant depressed adults were included. The primary outcome was mean change scores of depressive symptom on the depression rating scales, assessed with standardized mean differences. RESULTS: Five RCTs consisting of 154 patients met all inclusion and exclusion criteria. The overall pooled effect size in the primary and secondary efficacy analysis showed no significant effects of Pindolol plus SSRI therapy (standardized mean difference = -0.43, p = 0.24; OR = 1.92, p = 0.39, respectively). In terms of acceptability, there was no statistical difference in either tolerability or safety between the two groups (OR = 0.46, p = 0.40; OR = 0.90, p = 0.94, respectively). These estimates remained robust through several sensitivity and subgroup analyses, except 7.5 mg-qd Pindolol augmentation did show a significant benefit over 2.5-mg tid Pindolol augmentation. CONCLUSIONS: Pindolol augmentation may not be suitable for treatment-resistant depression patients with SSRI-resistant depression. However, once-daily high-dose Pindolol (7.5 mg qd) appears to show a promising benefit in these patients.
The antihypertensive drug pindolol attenuates long-term but not short-term binge-like ethanol consumption in mice.[Pubmed:27273539]
Addict Biol. 2017 May;22(3):679-691.
Alcohol dependence is a debilitating disorder with current therapies displaying limited efficacy and/or compliance. Consequently, there is a critical need for improved pharmacotherapeutic strategies to manage alcohol use disorders (AUDs). Previous studies have shown that the development of alcohol dependence involves repeated cycles of binge-like ethanol intake and abstinence. Therefore, we used a model of binge-ethanol consumption (drinking-in-the-dark) in mice to test the effects of compounds known to modify the activity of neurotransmitters implicated in alcohol addiction. From this, we have identified the FDA-approved antihypertensive drug Pindolol, as a potential candidate for the management of AUDs. We show that the efficacy of Pindolol to reduce ethanol consumption is enhanced following long-term (12 weeks) binge-ethanol intake, compared with short-term (4 weeks) intake. Furthermore, Pindolol had no effect on locomotor activity or consumption of the natural reward sucrose. Because Pindolol acts as a dual beta-adrenergic antagonist and 5-HT1A/1B partial agonist, we examined its effect on spontaneous synaptic activity in the basolateral amygdala (BLA), a brain region densely innervated by serotonin and norepinephrine-containing fibres. Pindolol increased spontaneous excitatory post-synaptic current frequency of BLA principal neurons from long-term ethanol-consuming mice but not naive mice. Additionally, this effect was blocked by the 5-HT1A/1B receptor antagonist methiothepin, suggesting that altered serotonergic activity in the BLA may contribute to the efficacy of Pindolol to reduce ethanol intake following long-term exposure. Although further mechanistic investigations are required, this study demonstrates the potential of Pindolol as a new treatment option for AUDs that can be fast-tracked into human clinical studies.
NO-dependent attenuation of TPA-induced immunoinflammatory skin changes in Balb/c mice by pindolol, heptaminol or ATRA, but not by verapamil.[Pubmed:27374093]
Oncotarget. 2016 Jul 26;7(30):47576-47585.
Recently a mouse skin carcinogenesis study reported that a beta-blocker carvedilol displayed antitumor-properties via antihyperplastic effects. However, the antihyperplastic mechanism is unclear as the beta-blocker is characterized with multiple pleiotropic effects including stimulation of endothelial NO release and verapamil-like calcium channel blocking activity. To investigate the nature and the origin of the antihyperplastic effects, we tested topical pretreatment with Pindolol, heptaminol, ATRA or verapamil against Balb/c mouse ear skin hyperplasia that was induced by TPA. We found that Pindolol, heptaminol or ATRA, but not verapamil, inhibited the TPA-induced immunoinflammatory skin changes in an NO-dependent manner, which included epidermal hyperplasia, skin edema and fibrosis. Furthermore, we also observed NO-dependent alleviation of the TPA-induced NK cell depletion in the ear tissues by heptaminol pretreatment. Together our results suggest that stimulation of NO generation from constitutive synthases may be primarily responsible for the reported antihyperplastic and NK cell-preserving effects of the beta-blockers, and that similar effects may be observed in other immunity normalizing compounds that also promote endothelial NO synthesis.
Electrophysiological and neurochemical evidence that pindolol has agonist properties at the 5-HT1A autoreceptor in vivo.[Pubmed:9630361]
Br J Pharmacol. 1998 May;124(1):206-12.
1. It has been hypothesized that 5-HT1A autoreceptor antagonists may enhance the therapeutic efficacy of SSRIs and other antidepressants. Although early clinical trials with the beta-adrenoceptor/5-HT1 ligand, Pindolol, were promising, the results of recent more extensive trials have been contradictory. Here we investigated the actions of Pindolol at the 5-HT1A autoreceptor by measuring its effect on 5-HT neuronal activity and release in the anaesthetized rat. 2. Pindolol inhibited the electrical activity of 5-HT neurones in the dorsal raphe nucleus (DRN). This effect was observed in the majority of neurones tested (10/16), was dose-related (0.2-1.0 mg kg(-1), i.v.), and was reversed by the 5-HT1A receptor antagonist, WAY 100635 (0.1 mg kg(-1), i.v.), in 6/7 cases tested. 3. Pindolol also inhibited 5-HT neuronal activity when applied microiontophoretically into the DRN in 9/10 neurones tested. This effect of Pindolol was current-dependent and blocked by co-application of WAY 100635 (3/3 neurones tested). 4. In microdialysis experiments. Pindolol caused a dose-related (0.8 and 4 mg kg(-1), i.v.) fall in 5-HT levels in dialysates from the frontal cortex (under conditions where the perfusion medium contained 1 microM citalopram). In rats pretreated with WAY 100635 (0.1 mg kg(-1), i.v.), Pindolol (4 mg kg(-1), i.v.) did not decrease, but rather increased 5-HT levels. 5. We conclude that, under the experimental conditions used in this study, Pindolol displays agonist effects at the 5-HT1A autoreceptor. These data are relevant to previous and ongoing clinical trials of Pindolol in depression which are based on the rationale that the drug is an effective 5-HT1A autoreceptor antagonist.
Antagonist properties of (-)-pindolol and WAY 100635 at somatodendritic and postsynaptic 5-HT1A receptors in the rat brain.[Pubmed:9504386]
Br J Pharmacol. 1998 Feb;123(3):449-62.
1. The aim of the present work was to characterize the 5-hydroxytryptamine1A (5-HT1A) antagonistic actions of (-)-Pindolol and WAY 100635 (N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl) cyclohexane carboxamide). Studies were performed on 5-HT1A receptors located on 5-hydroxytryptaminergic neurones in the dorsal raphe nucleus (DRN) and on pyramidal cells in the CA1 and CA3 regions of the hippocampus in rat brain slices. 2. Intracellular electrophysiological recording of CA1 pyramidal cells and 5-hydroxytryptaminergic DRN neurones showed that the 5-HT1A receptor agonist 5-carboxamidotryptamine (5-CT) evoked in both cell types a concentration-dependent cell membrane hyperpolarization and a decrease in cell input resistance. On its own, (-)-Pindolol did not modify the cell membrane potential and resistance at concentrations up to 10 microM, but it antagonized the 5-CT effects in a concentration-dependent manner. Similar antagonism of 5-CT effects was observed in the CA3 hippocampal region. (-)-Pindolol also prevented the 5-HT1A receptor-mediated hyperpolarization of CA1 pyramidal cells due to 5-HT (15 microM). In contrast, the 5-HT-induced depolarization mediated by presumed 5-HT4 receptors persisted in the presence of 3 microM (-)-Pindolol. 3. In the hippocampus, (-)-Pindolol completely prevented the hyperpolarization of CA1 pyramidal cells by 100 nM 5-CT (IC50=92 nM; apparent KB=20.1 nM), and of CA3 neurones by 300 nM 5-CT (IC50=522 nM; apparent KB= 115.1 nM). The block by (-)-Pindolol was surmounted by increasing the concentration of 5-CT, indicating a reversible and competitive antagonistic action. 4. Extracellular recording of the firing rate of 5-hydroxytryptaminergic neurones in the DRN showed that (-)-Pindolol blocked, in a concentration-dependent manner, the decrease in firing elicited by 100 nM 5-CT (IC50=598 nM; apparent KB= 131.7 nM) or 100 nM ipsapirone (IC50= 132.5 nM; apparent KB= 124.9 nM). The effect of (-)-Pindolol was surmountable by increasing the concentration of the agonist. Intracellular recording experiments showed that 10 microM (-)-Pindolol were required to antagonize completely the hyperpolarizing effect of 100 nM 5-CT. 5. In vivo labelling of brain 5-HT1A receptors by i.v. administration of [3H]-WAY 100635 ([O-methyl-3H]-N-(2-(4-(2-methoxyphenyl)-1 -piperazinyl)ethyl-N-(2-pyridyl)cyclo-hexane-carboxamide) was used to assess their occupancy following in vivo treatment with (-)-Pindolol. (-)-Pindolol (15 mg kg[-1]) injected i.p. either subchronically (2 day-treatment before i.v. injection of [3H]-WAY 100635) or acutely (20 min before i.v. injection of [3H]-WAY 100635) markedly reduced [3H]-WAY 100635 accumulation in all 5-HT1A receptor-containing brain areas. In particular, no differences were observed in the capacity of (-)-Pindolol to prevent [3H]-WAY 100635 accumulation in the DRN and the CAI and CA3 hippocampal areas. 6. Intracellular electrophysiological recording of 5-hydroxytryptaminergic DRN neurones showed that WAY 100635 prevented the hyperpolarizing effect of 100 nM 5-CT in a concentration-dependent manner (IC50=4.9 nM, apparent KB=0.25 nM). In CA1 pyramidal cells, hyperpolarization induced by 50 nM 5-CT was also antagonized by WAY 100635 (IC50 = 0.80 nM, apparent KB= 0.28 nM).
International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin).[Pubmed:7938165]
Pharmacol Rev. 1994 Jun;46(2):157-203.
It is evident that in the last decade or so, a vast amount of new information has become available concerning the various 5-HT receptor types and their characteristics. This derives from two main research approaches, operational pharmacology, using selective ligands (both agonists and antagonists), and, more recently, molecular biology. Although the scientific community continues to deliberate about the hierarchy of criteria for neurotransmitter receptor characterisation, there seems good agreement between the two approaches regarding 5-HT receptor classification. In addition, the information regarding transduction mechanisms and second messengers is also entirely consistent. Thus, on the basis of these essential criteria for receptor characterisation and classification, there are at least three main groups or classes of 5-HT receptor: 5-HT1, 5-HT2, and 5-HT3. Each group is not only operationally but also structurally distinct, with each receptor group having its own distinct transducing system. The more recently identified 5-HT4 receptor almost undoubtedly represents a fourth 5-HT receptor class on the basis of operational and transductional data, but this will only be definitively shown when the cDNA for the receptor has been cloned and the amino acid sequence of the protein is known. Although those 5-HT receptors that have been fully characterised and classified to date (and, hence, named with confidence) would seem to mediate the majority of the actions of 5-HT throughout the mammalian body, not all receptors for 5-HT are fully encompassed within our scheme of classification. These apparent anomalies must be recognised and need further study. They may or may not represent new groups of 5-HT receptor or subtypes of already known groups of 5-HT receptor. Even though the cDNAs for the 5-ht1E, 5-ht1F, 5-ht5, 5-ht6, and 5-ht7 receptors have been cloned and their amino acid sequence defined, more data are necessary concerning their operational and transductional characteristics before one can be confident of the suitability of their appellations. Therefore, it is important to rationalise in concert all of the available data from studies involving both operational approaches of the classical pharmacological type and those from molecular and cellular biology.(ABSTRACT TRUNCATED AT 400 WORDS)
Structural and conformational features determining selective signal transduction in the beta 3-adrenergic receptor.[Pubmed:7903415]
Mol Pharmacol. 1993 Dec;44(6):1094-104.
With respect to the beta 1- and beta 2-adrenergic receptors (ARs), the beta 3-AR induces specific physiological effects in a few target tissues and exhibits atypical pharmacological properties that distinguish it unambiguously from its counterparts. Therefore, the beta 3-AR represents a suitable model to study the molecular mechanism responsible for receptor subtype selectivity and specificity. Potent beta 3-AR ligands newly characterized in Chinese hamster ovary cells expressing the beta 3-AR were also evaluated in Chinese hamster ovary cells expressing beta 1- and beta 2-ARs and were classified into three groups according to their pharmacological properties. Among the beta 1/beta 2/beta 3 agonists BRL 37344 and LY 79771 exhibit beta 3 selectivity in stimulating adenylyl cyclase; among the beta 1/beta 2 antagonists displaying beta 3 agonistic effects ICI 201651 exhibits beta 3-AR binding selectivity, whereas among the beta 1/beta 2/beta 3 antagonist class bupranolol is the most efficient (but not selective) beta 3-AR antagonist. The structures of these ligands were simulated and compared using computer-generated molecular modeling. Structure-activity relationship analysis indicates that potent or selective beta 3-AR compounds, in addition to possessing a pharmacophore common to all beta-AR ligands, contain a long and bulky alkylamine substituent moiety, which is able to adopt and exchange extended and stacked conformations. Computerized three-dimensional models of the beta 1-, beta 2-, and beta 3-AR binding sites show that more bulky amino acid side chains point inside the groove of the beta 1 and beta 2 sites, compared with the beta 3 site, in a region implicated in signal processing. The long alkylamine chain of compounds behaving as beta 1/beta 2 antagonists and beta 3 agonists may thus adopt either a stacked conformation in the encumbered beta 1- and beta 2-AR sites, leading to antagonistic effects, or an extended conformation in the less encumbered beta 3 site, thus interacting with specific residues implicated in signal transduction.


