Prosaikogenin GCAS# 99365-23-8 |
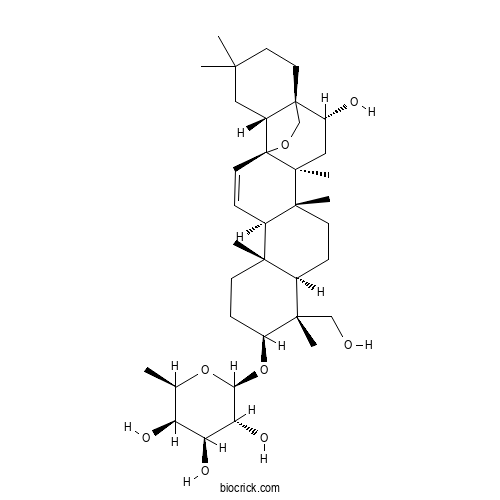
- Prosapogenin F
Catalog No.:BCX0606
CAS No.:99365-20-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 99365-23-8 | SDF | Download SDF |
| PubChem ID | 101596925.0 | Appearance | Powder |
| Formula | C36H58O8 | M.Wt | 618.84 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R,4S,5R,6R)-2-[[(1S,2R,4S,5R,8R,9R,10S,13S,14R,17S,18R)-2-hydroxy-9-(hydroxymethyl)-4,5,9,13,20,20-hexamethyl-24-oxahexacyclo[15.5.2.01,18.04,17.05,14.08,13]tetracos-15-en-10-yl]oxy]-6-methyloxane-3,4,5-triol | ||
| SMILES | CC1C(C(C(C(O1)OC2CCC3(C(C2(C)CO)CCC4(C3C=CC56C4(CC(C7(C5CC(CC7)(C)C)CO6)O)C)C)C)O)O)O | ||
| Standard InChIKey | WSSVJIGMYVWUJL-PSLKBDIJSA-N | ||
| Standard InChI | InChI=1S/C36H58O8/c1-20-26(39)27(40)28(41)29(43-20)44-25-10-11-31(4)21(32(25,5)18-37)8-12-33(6)22(31)9-13-36-23-16-30(2,3)14-15-35(23,19-42-36)24(38)17-34(33,36)7/h9,13,20-29,37-41H,8,10-12,14-19H2,1-7H3/t20-,21-,22-,23-,24-,25+,26+,27+,28-,29+,31+,32+,33-,34+,35-,36+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Prosaikogenin G Dilution Calculator

Prosaikogenin G Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6159 mL | 8.0796 mL | 16.1593 mL | 32.3185 mL | 40.3982 mL |
| 5 mM | 0.3232 mL | 1.6159 mL | 3.2319 mL | 6.4637 mL | 8.0796 mL |
| 10 mM | 0.1616 mL | 0.808 mL | 1.6159 mL | 3.2319 mL | 4.0398 mL |
| 50 mM | 0.0323 mL | 0.1616 mL | 0.3232 mL | 0.6464 mL | 0.808 mL |
| 100 mM | 0.0162 mL | 0.0808 mL | 0.1616 mL | 0.3232 mL | 0.404 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pterin-6-carboxylic acid
Catalog No.:BCX0799
CAS No.:948-60-7
- 17-Hydroxygracillin
Catalog No.:BCX0798
CAS No.:90308-85-3
- Kanzonol D
Catalog No.:BCX0797
CAS No.:155233-20-8
- (-)-Epipodophyllotoxin
Catalog No.:BCX0796
CAS No.:4375-07-9
- Magnoloside B
Catalog No.:BCX0795
CAS No.:116872-05-0
- O-Cymen-5-ol
Catalog No.:BCX0794
CAS No.:39660-61-2
- 7,4′-Dihydroxyhomoisoflavane
Catalog No.:BCX0793
CAS No.:148462-00-4
- Nor-rubrofusarin-6-O-β-D-gentiobioside
Catalog No.:BCX0792
CAS No.:245724-08-7
- N-noratherosperminine
Catalog No.:BCX0791
CAS No.:74606-53-4
- 6-hydroxyl kaempherol-3,6-O-diglucosyl-7-O-Glucuronic acid
Catalog No.:BCX0790
CAS No.:307950-53-4
- Yibeissine
Catalog No.:BCX0789
CAS No.:143502-51-6
- 3-[[6-Deoxy-2-O-[6-O-[3-(4-hydroxyphenyl)-1-oxo-2-propen-1-yl]-β-D-glucopyranosyl]-α-L-mannopyranosyl]oxy]-2-(3,4-dihydroxyphenyl)-7-(β-D-glucopyranosyloxy)-5-hydroxy-4H-1-benzopyran-4-one
Catalog No.:BCX0788
CAS No.:1575672-58-0
- Harmalan
Catalog No.:BCX0801
CAS No.:525-41-7
- Coreopsin
Catalog No.:BCX0802
CAS No.:499-29-6
- Quercetin 7-O-glucuronide
Catalog No.:BCX0803
CAS No.:38934-20-2
- Cortisone
Catalog No.:BCX0804
CAS No.:53-06-5
- Cortodoxone
Catalog No.:BCX0805
CAS No.:152-58-9
- Neoeuonymine
Catalog No.:BCX0806
CAS No.:33510-25-7
- Thymoquinone
Catalog No.:BCX0807
CAS No.:490-91-5
- Monascin
Catalog No.:BCX0808
CAS No.:21516-68-7
- guan-fu base I
Catalog No.:BCX0809
CAS No.:110225-59-7
- Acetyl Dopamine Dimer I
Catalog No.:BCX0810
CAS No.:315188-82-0
- Monascorubrin
Catalog No.:BCX0811
CAS No.:13283-90-4
- 16-Hydroxyhexadecanoic acid
Catalog No.:BCX0812
CAS No.:506-13-8
Production of Prosaikogenin F, Prosaikogenin G, Saikogenin F and Saikogenin G by the Recombinant Enzymatic Hydrolysis of Saikosaponin and their Anti-Cancer Effect.[Pubmed:35630731]
Molecules. 2022 May 19;27(10):3255.
The saponins of Bupleurum falcatum L., saikosaponins, are the major components responsible for its pharmacological and biological activities. However, the anti-cancer effects of prosaikogenin and saikogenin, which are glycoside hydrolyzed saikosaponins, are still unknown due to its rarity in plants. In this study, we applied two recombinant glycoside hydrolases that exhibit glycoside cleavage activity with saikosaponins. The two enzymes, BglPm and BglLk, were cloned from Paenibacillus mucilaginosus and Lactobacillus koreensis, and exhibited good activity between 30-37 degrees C and pH 6.5-7.0. Saikosaponin A and D were purified and obtained from the crude B. falcatum L. extract using preparative high performance liquid chromatography technique. Saikosaponin A and D were converted into saikogenin F via prosaikogenin F, and saikogenin G via Prosaikogenin G using enzyme transformation with high beta-glycosidase activity. The two saikogenin and two prosaikogenin compounds were purified using a silica column to obtain 78.1, 62.4, 8.3, and 7.5 mg of prosaikogenin F, Prosaikogenin G, saikogenin F, and saikogenin G, respectively, each with 98% purity. The anti-cancer effect of the six highly purified saikosaponins was investigated in the human colon cancer cell line HCT 116. The results suggested that saikosaponins and prosaikogenins markedly inhibit the growth of the cancer cell line. Thus, this enzymatic technology could significantly improve the production of saponin metabolites of B. falcatum L.
In vitro metabolism study of saikosaponin d and its derivatives in rat liver microsomes.[Pubmed:27052332]
Xenobiotica. 2017 Jan;47(1):11-19.
1. Saikosaponins, one of the representative bioactive ingredients in Radix Bupleuri, possess hepatoprotective, anti-inflammatory, antiviral, antitumor, and other pharmacological activities. Up to now, few studies focused on the further metabolism of saikosaponins and their secondary metabolites absorbed into the circulatory system. 2. To understand the in vivo efficacy of saikosaponin d, the in vitro metabolism of saikosaponin d, and its two derivatives formed in the gastrointestinal tract, Prosaikogenin G and saikogenin G was investigated in rat liver microsomes, respectively. 3. Fifteen metabolites were detected using high-performance liquid chromatography hybrid ion trap and time-of-flight mass spectrometry and triple-quadrupole mass spectrometry, and the predominant metabolic reactions were hydroxylation, carboxylation and combinations of these steps on the aglycone moiety. 4. The metabolic pathways of saikosaponin d, Prosaikogenin G, and saikogenin G were proposed in vitro and the results contribute to the understanding of saikosaponins in vivo metabolism.
A new saikogenin from the roots of Bupleurum bicaule.[Pubmed:24863358]
Chin J Nat Med. 2014 Apr;12(4):305-8.
AIM: To study the chemical constituents from the roots of Buleurum bicaule Helm (Apiaceae). METHOD: Silica gel, Sephadex LH-20, MPLC Rp-C18 column chromatography, and HPLC were used for isolation of compounds. The structures were elucidated on the basis of 1D- and 2D-NMR technology and HRESI-MS. Compounds were evaluated in vitro for their inhibitory ability against the proliferation of rat mesangial cells by the MTT method. RESULTS: Twelve compounds were isolated, and their structures were identified on the basis of their spectroscopic and physico-chemical properties as 13, 28-epoxy-olean-11-en-3-one (1), saikogenin E (2), saikogenin G (3), 11alpha-methoxy-3beta, 16beta, 23, 28-tetrahydroxyolean-12-ene (4), saikogenin D (5), prosaikogenin F (6), prosaikogenin A (7), Prosaikogenin G (8), prosaikogenin D (9), laccaic acid (10b), methyl gallate (11), and ethyl gallate (12). Compounds 1, 2, 7, 8, and 10 were observed to have inhibitory activity against mesangial cell proliferationin to different degrees. CONCLUSION: Compound 1, 8, and 10 exhibit significant inhibitory effects on rat mesangial cell proliferation induced by Ang II.
Saikosaponin derivatives from Bupleurum wenchuanense.[Pubmed:7764030]
Phytochemistry. 1993 Jul;33(5):1197-1205.
From the roots of Bupleurum wenchuanense 14 derivatives of saikosaponin were isolated and identified as 2"-O-beta-D-xylopyranosylsaikosaponin b2, 3",6"-O O-diacetylsaikosaponin b2, 2"-O-beta-D-glucopyranosylsaikosaponin b2, saikosaponin b2, 6"-O-acetylsaikosaponin b2, saikosaponin d, 2"-O-acetylsaikosaponin d, 3"-O-acetylsaikosaponin d,6"-O-acetylsaikosaponin d, 16-epichikusaikoside, Prosaikogenin G, saikosaponin a,2"-O-acetylsaikosaponin a and 3"-O-acetylsaikosaponin a. The first two compounds are new derivatives of saikosaponin and this is the first isolation of Prosaikogenin G from a plant. Their complete 1H and 13C NMR assignments were made by using a combination of 2D NMR techniques (DQF-COSY, HOHAHA, ROESY, HETCOR, HMQC and HMBC). Some of the compounds showed cytotoxic activity against the P-388 cell line.
Effects of saikosaponin metabolites on the hemolysis of red blood cells and their adsorbability on the cell membrane.[Pubmed:2632076]
Chem Pharm Bull (Tokyo). 1989 Dec;37(12):3306-10.
The hemolytic properties and the adsorbability on red blood cells of saikosaponin a, saikosaponin d and 13 metabolites formed in the alimentary tract were investigated. Among these compounds, saikosaponin d and its intestinal product, Prosaikogenin G, which possess an alpha-hydroxyl function at C16, showed the strongest hemolytic activity at the dose range of 1.0 to 5.0 micrograms/ml. Saikosaponin a and its intestinal product, prosaikogenin F, which possess a beta-hydroxyl function at C16, showed activity above 10 micrograms/ml. In this case, the monoglycoside, prosaikogenin F, showed the stronger activity than the diglycoside, saikosaponin a. Among the gastric products whose ether ring was cleaved to produce a carbinol, the monoglycosides, prosaikogenin A and prosaikogenin H, showed a slight activity above 25 micrograms/ml, and the saikogenins except saikogenin A were inactive. Saikogenin A, however, had hemolytic activity at a dose of 15 micrograms/ml. The adsorbabilities of these compounds on red blood cell membranes closely paralleled their degrees of hemolytic activity.
Corticosterone secretion-inducing activity of saikosaponin metabolites formed in the alimentary tract.[Pubmed:2611932]
Chem Pharm Bull (Tokyo). 1989 Oct;37(10):2736-40.
The corticosterone secretion-inducing activities of saikosaponin a, saikosaponin c and saikosaponin d, isolated from the root of Bupleurum falcatum L., and 27 metabolites formed in the murine alimentary tract were studied in mice. Serum corticosterone was determined by high-performance liquid chromatography (HPLC). Intraperitoneal administration of saikosaponin a and its intestinal metabolite, prosaikogenin F, showed corticosterone secretion-inducing activity at a dose of 0.1 mmol/kg, and maximally increased it at a dose of 0.4 mmol/kg. On the other hand, the genuine sapogenin, saikogenin F, was inactive. Saikosaponin b1 and saikosaponin g, gastric metabolites of saikosaponin a, and their intestinal metabolites, prosaikogenin A, prosaikogenin H, saikogenin A and saikogenin H, were also inactive. Serum corticosterone was increased by the administration of saikosaponin d and its intestinal metabolite, Prosaikogenin G, at a dose of 0.04 mmol/kg, and it reached the maximal level at the dose of 0.1 mmol/kg. Saikogenin G also showed a slight activity. A gastric metabolite of saikosaponin d, saikosaponin b2, and its intestinal metabolites, prosaikogenin D and saikogenin D, were inactive. In the experiments on saikosaponin c and its metabolites, saikosaponin c was inactive but its intestinal metabolites, especially prosaikogenin E-2, showed activity almost equal to that of saikosaponin a. Saikosaponin h and saikosaponin i, gastric metabolites of saikosaponin c, were also inactive, but their prosaikogenins showed slight activities. When these compounds were orally administered, their corticosterone secretion-inducing activities were similar to those obtained in the intraperitoneal experiment. These results suggest that a proper polar balance between the sugar moiety and the aglycone is important for the corticosterone secretion-inducing activity of saikosaponins and their metabolites.


